Novel Therapeutic Avenues in Myeloma: Changing the Treatment Paradigm
Our better understanding of the complex interaction of multiple myeloma (MM) cells with their bone marrow microenvironment and the signaling pathways that are dysregulated in this process has resulted in a dramatic increase in the therapeutic agents available for this disease. A number of these new agents have demonstrated significant activity in patients with MM. Over the past 5 years, three drugs have received approval from the US Food and Drug Administration for therapy in MM—bortezomib, thalidomide, and lenalidomide. To date, the choice of therapy for MM is not individualized according to the biologic characteristics of the disease, but future studies should enable us to identify patients who may benefit most from certain therapeutic interventions, and thus develop individualized therapy for MM. In this review, we will present some of the treatment algorithms currently developed for patients with MM and focus on established advances in therapy, specifically with thalidomide, bortezomib, and lenalidomide. We will also discuss some of the emerging novel therapeutic agents showing promise in phase I/II clinical trials in MM.
Our better understanding of the complex interaction of multiple myeloma (MM) cells with their bone marrow microenvironment and the signaling pathways that are dysregulated in this process has resulted in a dramatic increase in the therapeutic agents available for this disease. A number of these new agents have demonstrated significant activity in patients with MM. Over the past 5 years, three drugs have received approval from the US Food and Drug Administration for therapy in MM-bortezomib, thalidomide, and lenalidomide. To date, the choice of therapy for MM is not individualized according to the biologic characteristics of the disease, but future studies should enable us to identify patients who may benefit most from certain therapeutic interventions, and thus develop individualized therapy for MM. In this review, we will present some of the treatment algorithms currently developed for patients with MM and focus on established advances in therapy, specifically with thalidomide, bortezomib, and lenalidomide. We will also discuss some of the emerging novel therapeutic agents showing promise in phase I/II clinical trials in MM.
Multiple myeloma (MM) is characterized by the presence of a malignant clone of plasma cells in the bone marrow and a monoclonal protein in the serum or urine. It represents a spectrum of disease from monoclonal gammopathy of undetermined significance (MGUS) to smoldering myeloma and symptomatic, active MM. MGUS and smoldering myeloma are asymptomatic, with MGUS requiring no therapeutic intervention other than observation. Similarly, the mainstay of management for smoldering myeloma is careful observation, although the selected use of erythropoietic agents for mild anemia, bisphosphonates for osteopenia, and participation in clinical trials are appropriate considerations in selected patients. Conversely, active MM characterized by the presence of hypercalcemia (C), renal insufficiency (R), anemia (A), or lytic bone lesions (B)-summarized by the acronym CRAB-requires treatment to prevent worsening complications and end organ damage.[1,2]
The rate of progression of MGUS to MM is 0.6 to 3% per year, with many patients never developing any symptoms from this plasma cell dyscrasia.[3] Patients with smoldering disease are at higher risk, with progression to active disease occurring from between 1 to 2 years to a median of 5 to 7 years in some series, reflecting considerable heterogeneity of disease biology in this population.
Once active MM develops, proper staging and evaluation is required to determine prognostic factors that may affect the choice of therapeutic agents to be used. Several factors have been associated with poor prognosis in MM, including decreased serum albumin, increased beta-2-microglobulin, interleukin (IL)-6, C-reactive protein, plasma cell labeling index, the presence of circulating plasma cells, and cytogenetic abnormalities.[4-8] Karyotyping is a key prognostic tool in MM, as it identifies numerical chromosomal abnormalities. Specifically, it is a sensitive technique of determining abnormalities of chromosome 13.[9]
More broadly, MM can be classified into hyperdiploid and nonhyperdiploid forms.[9] Aneuploidy occurs frequently in MM, with monosomies (including 13, 14, 16, and 22) being more frequent than trisomies (including 3, 5, 7, 9, 11, 15, 19, and 21). Hyperdiploid MM is associated with multiple trisomies and a low incidence of IgH translocations.[9] Nonhyperdiploid MM is characterized by a very high prevalence of IgH translocations, and five recurrent chromosomal partners are involved in IgH translocations occuring in about 40% of the patients,[10] including 11q13 (cyclin D1), 6p21 (cyclin D3), 4p16 (fibroblast growth factor receptor 3 [FGFR3] and multiple myeloma SET domain [MMSET]), 16q23 (c-maf), and 20q11 (mafB).[9,11] Identifying these changes has enhanced our understanding of the pathogenesis of MM, and in turn this has led to the development of agents that specifically target these abnormalities, such as FGFR3 inhibitors, which are discussed below.
Critically, the advances in our understanding of the complex interaction of the MM cells with the bone marrow microenvironment and the signaling pathways that are dysregulated in this process have been associated with a surge in the investigational agents tested for this disease. A number of these agents have demonstrated efficacy in MM patients, and specifically, in the past 5 years three of these drugs have received US Food and Drug Administration (FDA) approval for the treatment of MM: bortezomib (Velcade), thalidomide (Thalomid), and lenalidomide (Revlimid). Therapy for MM can now be tailored depending on the clinical disease characteristics. Future studies determining sensitive and specific molecular markers of response/resistance to specific anti-MM therapies should enable us to better identify patients who would benefit most from certain therapeutic interventions, and thus help develop individualized approaches to therapy for the disease.
In this review, we will focus on treatment algorithms currently developed for patients with MM and focus on established advances in therapy for MM, including the use of thalidomide, bortezomib, and lenalidomide. We will also discuss other novel therapeutic agents showing promising activity in early-phase clinical trials in MM.
Treatment Overview
After 20 years of relative inertia, where alkylator-based cytotoxic chemotherapy and glucocorticoids remained the backbone of therapy, the use of anthracyclines, the advent of high-dose therapy supported by stem cell transplant, and then the introduction of bisphosphonates punctuated the 1980s and 1990s.[12] Since the promising results of thalidomide for the treatment of relapsed MM reported in 1999,[13] and the accelerated approval of bortezomib for relapsed and refractory MM in 2003, the treatment algorithm has been continuously changing. As change has come to the relapse setting, upfront therapy has also changed. Clinical trials remain a cornerstone of management throughout the course of the illness; studies are of critical importance not only to determine the combinations and sequences that will lead to higher response rates and improved survival, but also to test the activity of new agents in MM.
Thalidomide
Thalidomide represents the first novel agent to be tested in MM, catalyzing the advance of novel therapeutic agents in this disease. Thalidomide was first introduced as a sedative and hypnotic drug in the 1950s. Tragically, it was later found to be a teratogenic agent that caused malformations in over 10,000 newborns as well as a similar number of still births.[14] After its withdrawal from the market, thalidomide continued to be studied in highly selected settings and was found to have antiangiogenic activity.[15] In 1999, it was tested as a single agent in patients with relapsed MM, producing an overall response rate of 25%.[16]
The major toxicities of thalidomide include peripheral neuropathy and deep-vein thrombosis (DVT).[17] Other toxicities include fatigue, somnolence, constipation, rash, Stevens-Johnson syndrome, and hepatic dysfunction.[17] Several studies then tested the combination of thalidomide with other drugs such as dexamethasone and chemotherapeutic agents, and led to responses as high as 65% in these patients.[18-21]
Thalidomide has been tested in combination with dexamethasone in the upfront setting in large phase III trials in patients who are transplant candidates, and in combination with MP (melphalan [Alkeran], prednisone) for non-transplant candidates. For transplant candidates, the combination of thalidomide and high-dose dexamethasone was compared to high-dose dexamethasone alone for newly diagnosed MM patients, resulting in a 63% response rate in the thalidomide/dexamethasone arm vs 41% in the dexamethasone arm. This study led to the FDA approval of thalidomide in combination with dexamethasone in newly diagnosed patients in 2006.
Other phase III trials were performed in elderly patients who were not candidates for autologous stem cell transplant. A randomized study of MPT (melphalan, prednisone, thalidomide) compared to MP demonstrated that patients treated with MPT had higher response rates and longer event-free survival than patients treated with MP alone.[22] Subsequently, Facon et al presented a large phase III trial of MPT compared to MP or high-dose chemotherapy and stem cell transplantation in elderly patients from 65 to 75 years of age and showed that patients treated with MPT had a longer overall survival of 54 months compared to 32 months for MP and 39 months for transplant.[23] Together, these data indicate that thalidomide used in combination with other agents has led to strong clinical activity in the upfront or relapsed setting.
Bortezomib
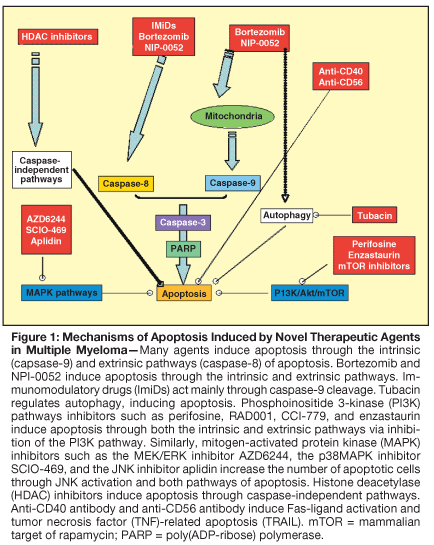
Bortezomib (PS-341) is an inhibitor of the 26S proteasome that leads to significant induction of apoptosis in MM cells by targeting the intrinsic and extrinsic apoptotic pathways, NF-kappaB pathway, and many other signaling pathways, without significantly affecting normal hematopoietic cells.[24] The SUMMIT trial (Study of Uncontrolled Multiple Myeloma managed with proteasome Inhibition Therapy) was one of the pivotal studies that showed the activity of single-agent bortezomib, which produced an overall response rate of 35% in 202 patients with relapsed MM.[25] The main side effects of bortezomib included reversible thrombocytopenia and peripheral neuropathy.[26]
A phase III trial comparing single-agent bortezomib to high-dose dexamethasone in patients with relapsed MM (APEX study, Assessment of Proteasome Inhibition for Extending Remissions) showed an overall response rate of 38% in the bortezomib arm compared to only 18% in the high-dose dexamethasone arm.[27] The SUMMIT study led to the accelerated approval of bortezomib in 2003, with full approval based on the APEX study in 2005, reflecting a remarkable and groundbreaking time line for rapid drug development in MM. Studies using the combination of bortezomib with other therapeutic agents showed increased activity. The CREST (Clinical Response and Efficacy Study of bortezomib in the Treatment of myeloma) trial-a phase II study randomizing patients to higher (1.3 mg/m2) or lower (1.0 mg/m2) doses of bortezomib in combination with dexamethasone-revealed positive response rates (33% with low-dose bortezomib alone, 44% with low-dose bortezomib/dexamethasone, 50% with high-dose bortezomib, and 62% with high-dose bortezomib/dexamethasone).[28]
Other combinations tested in clinical trials included chemotherapies as well as combinations with other novel agents, such as thalidomide or lenalidomide.[29] The combination of bortezomib, thalidomide, and dexamethasone (VTD) in patients with relapsed MM showed an overall response rate of 70% including near-complete responses in 16%.[29] High response levels were also observed in studies of patients with previously untreated MM. Single-agent bortezomib showed an overall response rate of 40%, with 10% complete responses, in a phase II study of 66 patients with MM.[30] The combination of bortezomib and dexamethasone led to an overall response rate of 66% to 88% in another phase II trial of newly diagnosed MM.[26,31-33] In addition, the combination of melphalan, prednisone, and bortezomib (MPV) in non-transplant candidates resulted in an overall response rate of 89%.[34] A phase III trial randomizing patients to MPV or MP was recently completed, and results are awaited with interest.
Lenalidomide
Lenalidomide (CC5013; IMiD3) is a potent thalidomide analog belonging to the immunomodulatory (IMiD) class of drugs. The first studies performed with lenalidomide (25 mg daily for 3 weeks with 1 week off) demonstrated response rates of 25% to 35% in patients with relapsed MM.[35] The main side effects of lenalidomide include cytopenias, rash, and DVT.[21]
Two large randomized phase III studies were conducted in the United States and Europe comparing lenalidomide and dexamethasone to dexamethasone and placebo for patients with relapsed or relapsed and refractory MM (MM-009 and MM-010). These trials were comparable in results and showed response rates with the lenalidomide/dexamethasone combination greater than twice the response rates seen with dexamethasone alone.[36,37] This led to the discontinuation of the trials because they exceeded the prespecified efficacy values as determined by an independent Data Monitoring Committee. Based on these studies, lenalidomide received FDA approval for the treatment of relapsed MM in June 2006.
Similarly exciting responses have been reported in previously untreated patients with MM. A phase II study of the combination of lenalidomide and dexamethasone in newly diagnosed MM patients showed an overall response rate of 91%.[38] A large phase III study of lenalidomide in combination with high-dose dexamethasone compared to lenalidomide with a low dose of dexamethasone was recently presented in abstract form and showed that the combination with high-dose dexamethasone resulted in greater toxicities, including a higher risk for thromboembolism and arrhythmias. The response rates in that study have not yet been presented. In non-transplant candidates, a recent study of the combination of lenalidomide, melphalan, and prednisone was associated with a response rate of 86%.[39] Studies to test lenalidomide as maintenance therapy or in combination with other agents are ongoing.
Other Novel Agents in Clinical Trials
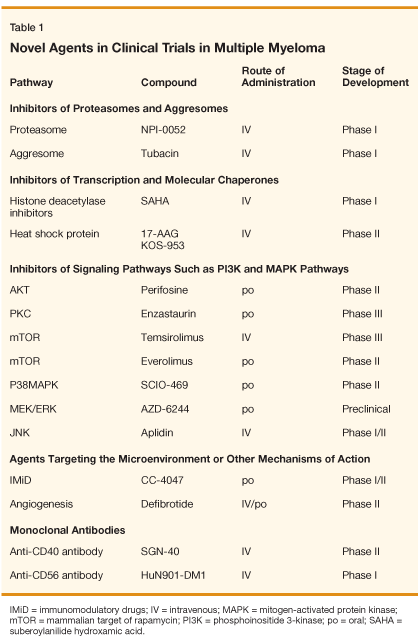
Multiple other agents are currently being tested in phase I and II clinical trials, and many hold promise. These include (1) inhibitors of the proteasome and aggresome, (2) modulators of transcription and inhibitors of molecular chaperones, (3) inhibitors of signaling pathways such as phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK), (4) other IMiDs (eg, CC-4047 [Actimid]), (5) agents with properties related to modulation of tumor-microenvironment interactions (eg, defibrotide), and (6) monoclonal antibodies against surface antigens of MM cells (eg, CD40, CD56). Overall, there are more than 30 other agents being tested in preclinical studies in MM.
Proteasome and Aggresome Inhibitors
The proteasome/ubiquination pathway plays a key role in the control of MM cell survival, and there appears to be a therapeutic index for inhibition of this target in some cancers, particularly MM. This is reflected in the fact that bortezomib-the first-in-class proteasome inhibitor-has shown remarkable clinical efficacy in MM. Consequently, other proteasome inhibitors are now in development, in an effort to recapitulate and hopefully expand the spectrum of clinical activity of this drug class.
One of these second-generation proteasome inhibitors is the oral agent NPI-0052.[40] This agent has shown in vitro activity by inducing apoptosis and inhibiting the growth of MM cell lines, even those resistant to bortezomib therapy.[40] NPI-0052 has also demonstrated in vivo activity in animal MM models. In addition, the combination of NPI-0052 and bortezomib proved to be synergistic, indicating different mechanisms of activity for these two proteasome inhibitors.[40] A phase I clinical trial of NPI-0052 in relapsed MM is planned.
Misfolded proteins are disposed of inside the cell by several processes including proteasome degradation. Another mechanism of protein disposal involves the sequestration of aggregated proteins into spherical aggresomes for further processing. Aggresome inhibition may be an important strategy for treatement of MM. Tubacin is a small molecule that directly inhibits the function of histone deacetylase 6 (HDAC6), which plays an essential role in aggresome activity. Tubacin inhibits the growth of MM cells in vitro and in vivo and is synergistic with bortezomib in its cytotoxic activity on MM cells.[41] Although tubacin in itself is unlikely to be clinically usable, other compounds in this class are in development, and clinical studies are anticipated with keen interest.
Modulators of Transcription and Molecular Chaperones
Transcription modulators such as HDAC inhibitors (suberoylanilide hydroxamic acid [SAHA], NVP-LAQ824, and NVP-LBH589) are being tested in MM. SAHA has demonstrated in vitro and in vivo cytotoxic activity in MM cells lines and patient samples, as well as strong antiproliferative activity when combined with other agents such as bortezomib.[42] A phase I clinical trial of SAHA in MM has shown manageable toxicity and some modest activity.[43] Combination studies with bortezomib are planned.
Heat shock protein 90 (Hsp90) inhibitors such as geldanamycin and 17-AAG modulate the stress-induced antiapoptotic response in MM cells[44] and have demonstrated in vitro and in vivo antitumor activity alone and in combination with other agents active in MM, specifically bortezomib.[44] Phase I clinical trials of KOS-953-which consists of 17-AAG provided in a Cremophor-based formulation for IV administration-in MM have shown good tolerability with disease stabilization and minor responses as a single agent for treatment of patients with relapsed and refractory MM. Other Hsp90 inhibitors include IPI-504, which is also being tested in a phase I clinical trial in MM and is associated with excellent tolerability but no clinical responses at doses tested to date.[45,46] Excitingly, KOS-953 combined with bortezomib has demonstrated responses even in bortezomib-resistant patients in an ongoing phase I/II trial in patients with relapsed and refractory MM.[45,47] Phase III trials of this combination are planned.
Inhibitors of the PI3K Pathway
The AKT inhibitor perifosine (NSC 639966) is an orally active alkyl-phosphocholine compound that affects membrane permeability and signal transduction.[48] A phase II clinical trial of perifosine with or without dexamethasone in patients with relapsed and refractory MM was recently reported; the drug showed activity, with 69% of patients achieving a response and/or stabilization of disease.[49] Another phase II trial of the combination of perifosine plus bortezomib with or without dexamethasone is currently underway.
Mammalian target of rapamycin (mTOR) inhibitors such as rapamycin and rapamycin analogs, including temsirolimus (CCI-779, Torisel) and everolimus (RAD001, Certican), have demonstrated in vitro and in vivo activity in MM cell lines and animal models.[50,51] The combination of rapamycin with active agents in MM such as lenalidomide, bortezomib, and 17-AAG have demonstrated synergistic activity in vitro.[52,53] In addition, rapamycin appears to target the bone marrow microenvironment by inhibiting angiogenesis and osteoclast formation in MM in vitro.[53] Both temsirolimus and everolimus have been tested clinically and showed some activity with stable disease and minor response in a subset of relapsed MM patients. These findings have led to the design of studies using these agents in combination with other active agents in MM, including a planned phase I/II trial of CCI-779 in combination with bortezomib, and everolimus in combination with lenalidomide.
Enzastaurin (LY317615) is an oral PKC-beta inhibitor, producing downstream inhibition of Akt54. In MM, enzastaurin has demonstrated specific inhibition of PKC isoforms and Akt activation along with inducing cytotoxicity and apoptosis in MM cells in vitro and in vivo.[54] In addition, enzastaurin inhibited MM cell adhesion, as well as vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF)-1-triggered MM cell migration, and angiogenesis.[54] Clinical trials in combination with bortezomib are planned.
Inhibitors of MAPK Pathways
The specific MEK pathway inhibitor AZD6244 has been tested in preclinical models in MM and induced inhibition of growth and cytotoxicity in MM cells even in the presence of cytokines/growth factors such as IL-6 and IGF-1 that induce MEK/ERK activation.[11,55] A phase II trial of single-agent AZD6244 is planned in 2007 for patients with relapsed/refractory MM.
The p38MAPK inhibitor SCIO-469 was first studied in clinical trials in rheumatoid arthritis and has shown in vitro activity in MM cells when cocultured with bone marrow stromal cells.[56] The combination of SCIO-469 and bortezomib demonstrated synergistic activity in vitro and in vivo.[56] A phase II trial of SCIO-469 alone or in combination with bortezomib in patients with relapsed MM showed stable disease in 24% with single-agent SCIO-469; the combination resulted in a response rate of 32%, including responses in patients in whom bortezomib had failed.[57]
The JNK inhibitor plitidepsin (Aplidin) is a naturally occurring, cyclic depsipeptide isolated from the marine tunicate Aplidium albicans. This agent exhibits very promising antitumor effects both in vitro and in vivo, and it is now in phase II clinical trials for a variety of solid and hematologic tumors. The exact mechanisms of action are unclear, but plitidepsin-mediated cytotoxicity has been shown to be dependent on sustained activation of JNK.[61-63] It exhibits strong apoptotic effects on MM cell lines and patient cells by triggering JNK, Fas, and mitochondrial-mediated signaling pathways.[64]
Lopez-Martin and colleagues reported in abstract form that in phase I and II trials of 215 and 112 patients, respectively, plitidepsin was generally well tolerated, with its major dose-limiting toxicity being adverse musculoskeletal events including increased creatine kinase, myalgia, and weakness.[65] Tumor shrinkage and long-lasting disease stabilization were reported in patients with metastatic colorectal, renal, and neuroendocrine tumors. Studies to date in MM have shown minor and partial response rates of approximately 30% in relapsed and refractory MM patients in an ongoing phase II study,[58] with manageable toxicity. Combination trials with bortezomib are planned.
Other Targeted Agents
• Other IMiDs-Based on the significant clinical activity of lenalidomide in MM, other IMiDs have been developed. CC-4047 (Actimid) has been tested as a single agent in a phase I clinical trial of 24 patients with relapsed MM. Treatment resulted in a greater than 25% reduction in paraprotein in 67% of patients, a greater than 50% reduction in paraprotein in 13 patients (54%), and complete remission in 4 (17%) of the 24 patients.[59] Toxicities included myelosuppression and DVT but were generally manageable. Phase II studies with and without dexamethasone are planned.
• Defibrotide-Drugs targeting the micronenvironment alone are of interest, not least because they may provide an important platform to not only expand the therapeutic index but also reduce toxicties as part of combination regimens. One example is defibrotide, an orally bioavailable oligonucleotide with protective effects on endothelial cells but without significant systemic anticoagulant effects and bleeding risk. Defibrotide has shown minimal inhibitory effect on MM cells in vitro but targets tumor-microenvironmental interactions and sensitizes MM to cytotoxic chemotherapy.[60] Moreover, it increases the response of human MM xenografts in SCID/NOD mice to melphalan, cyclophosphamide, and dexamethasone,[60] possibly by abrogating MM cell interactions with BM stromal cells.
The ability of defibrotide to protect against thrombosis, while potentially enhancing the sensitivity of MM cells to other therapies has provided the rationale for an ongoing clinical trial to determine the efficacy and safety of MPTD (melphalan, prednisone, thalidomide, and defibrotide) as salvage treatment in patients with relapsed/refractory MM.[61] In this trial, MPTD has provided promising evidence of antitumor activity in relapsed and refractory MM, with manageable toxicities. The absence of significant nonhematologic toxicity with this regimen, including no DVT and no neuropathy to date, is especially encouraging.
• Monoclonal Antibodies-Monoclonal antibodies have become an important component of therapy in patients with other B-cell neoplasms, best reflected by the use of the anti-CD20 antibody rituximab (Rituxan). CD40 is highly expressed on MM cells and targeted humanized anti-CD40 antibodies such as SGN-40 have demonstrated cytotoxicity in MM cell lines, even those resistant to conventional therapies.[62] Phase I trials of SGN-40 demonstrated the safety of this agent in MM with promising responses,[63,64] and phase II trials are currently underway. Other antibodies being tested in clinical trials in MM include the anti-CD56 antibody huN901-DM1.
Conclusions
In summary, the past decade has marked a new era in the treatment of MM. This paradigm shift of the use of novel targeted agents instead of or in combination with chemotherapy has become an emerging standard of care for newly diagnosed and relapsed/refractory MM. Indeed, the combination of novel agents with chemotherapeutic agents and/or dexamethasone has demonstrated high response rates with complete remission rates as high as those achieved in the stem cell transplant setting.
The future holds many more challenges for the treatment of MM. Overcoming these challenges will include combinations of agents that achieve higher responses and longer survival, individualized therapies that are based on genetic and molecular abnormalities present in MM patients, and clinical trials to test the benefit of novel agents in comparison and in addition to high-dose chemotherapy supported by stem cell transplantation, as well as other conventional approaches. Together, these therapies should lead to higher response rates, more durable duration of response, less toxicity, and prolonged survival, making MM an increasingly chronic and treatable disease.
References:
1. Kyle RA, Rajkumar SV: Monoclonal gammopathy of undetermined significance. Clin Lymphoma Myeloma 6:102-114, 2005.
2. Kyle RA, Gertz MA, Witzig TE, et al: Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78:21-33, 2003.
3. Kyle RA, Therneau TM, Rajkumar SV, et al: Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo Clinic series 25 years later. Mayo Clin Proc 79:859-866, 2004.
4. Kumar S, Rajkumar SV, Greipp PR, et al: Cell proliferation of myeloma plasma cells: comparison of the blood and marrow compartments. Am J Hematol 77:7-11, 2004.
5. Rajkumar SV, Kyle RA: Angiogenesis in multiple myeloma. Semin Oncol 28:560-564, 2001.
6. Nowakowski GS, Witzig TE, Dingli D, et al: Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 106:2276-2279, 2005.
7. Greipp PR, San Miguel J, Durie BG, et al: International staging system for multiple myeloma. J Clin Oncol 23:3412-3420, 2005.
8. Fonseca R, Debes-Marun CS, Picken EB, et al: The recurrent IgH translocations are highly associated with nonhyperdiploid variant multiple myeloma. Blood 102:2562-2567, 2003.
9. Chng WJ, Fonseca R: Genomics in multiple myeloma: Biology and clinical implications. Pharmacogenomics 6:563-557, 2005.
10. Bergsagel PL, Kuehl WM: Critical roles for immunoglobulin translocations and cyclin D dysregulation in multiple myeloma. Immunol Rev 194:96-104, 2003.
11. Hideshima T, Bergsagel PL, Kuehl WM, et al: Advances in biology of multiple myeloma: Clinical applications. Blood 104:607-618, 2004.
12. Kyle RA: Five decades of therapy for multiple myeloma: A paradigm for therapeutic models. Leukemia 19:910-912, 2005.
13. Rajkumar SV, Kyle RA: Thalidomide in the treatment of plasma cell malignancies. J Clin Oncol 19:3593-3595, 2001.
14. Rajkumar SV: Thalidomide: Tragic past and promising future. Mayo Clin Proc 79:899-903, 2004.
15. Hideshima T, Chauhan D, Shima Y, et al: Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 96:2943-2950, 2000.
16. Singhal S, Mehta J, Desikan R, et al: Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341:1565-1571, 1999.
17. Ghobrial IM, Rajkumar SV: Management of thalidomide toxicity. J Support Oncol 1:194-205, 2003.
18. Rajkumar SV, Fonseca R, Dispenzieri A, et al: Thalidomide in the treatment of relapsed multiple myeloma. Mayo Clin Proc 75:897-901, 2000.
19. Weber D, Rankin K, Gavino M, et al: Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol 21:16-19, 2003.
20. Rajkumar SV, Hayman S, Gertz MA, et al: Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol 20:4319-4323, 2002.
21. Kumar S, Rajkumar SV: Thalidomide and lenalidomide in the treatment of multiple myeloma. Eur J Cancer 42:1612-1622, 2006.
22. Palumbo A, Bringhen S, Caravita T, et al: Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: Randomised controlled trial. Lancet 367:825-831, 2006.
23. Facon T, Mary, J, Harousseau, J, et al: Superiority of melphalan-prednisone (MP) + thalidomide (THAL) over MP and autologous stem cell transplantation in the treatment of newly diagnosed elderly patients with multiple myeloma (abstract 1). J Clin Oncol 24(18S):1s, 2006.
24. Richardson PG, Hideshima T, Anderson KC: Bortezomib (PS-341): A novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control 10:361-369, 2003.
25. Richardson PG, Barlogie B, Berenson J, et al: A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348:2609-2617, 2003.
26. Richardson PG, Mitsiades C, Hideshima T, et al: Bortezomib: Proteasome inhibition as an effective anticancer therapy. Annu Rev Med 57:33-47, 2006.
27. Richardson PG, Sonneveld P, Schuster MW, et al: Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352:2487-2498, 2005.
28. Jagannath S, Barlogie B, Berenson J, et al: A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 127:165-172, 2004.
29. Richardson PG, Mitsiades C, Ghobrial I, et al: Beyond single-agent bortezomib: Combination regimens in relapsed multiple myeloma. Curr Opin Oncol 18:598-608, 2006.
30. Anderson K, Richardson P, Chanan-Khan A, et al: Single-agent bortezomib in previously untreated multiple myeloma (MM): Results of a phase II multicenter study (abstract 7504). J Clin Oncol 24(18S):423s, 2006.
31. Jagannath S, Richardson PG, Barlogie B, et al: Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica 91:929-934, 2006.
32. Jagannath S, Durie BG, Wolf J, et al: Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol 129:776-783, 2005.
33. Harousseau JL, Attal M, Leleu X, et al: Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: Results of an IFM phase II study. Haematologica 91:1498-1505, 2006.
34. Mateos MV, Hernandez JM, Hernandez MT, et al: Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood 108:2165-2172, 2006.
35. Richardson PG, Mitsiades C, Hideshima T, et al: Lenalidomide in multiple myeloma. Expert Rev Anticancer Ther 6:1165-1173, 2006.
36. Dimopoulos MA, Spencer A, Attal M, et al: Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): Results of a phase 3 study (MM010) (abstract 6). Blood 106:6a, 2005.
37. Weber D, Chen C, Niesvizky M, et al: Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): Results of a North American phase III study (MM-009) (abstract 7521). J Clin Oncol 24(18S):427s, 2006.
38. Rajkumar SV, Hayman SR, Lacy MQ, et al: Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood 106:4050-4053, 2005.
39. Palumbo A, Falco P, Falcone A, et al: Oral Revlimid plus melphalan and prednisone (R-MP) for newly diagnosed multiple myeloma: Results of a multicenter phase I/II study (abstract 800). Blood 108:240a, 2006.
40. Chauhan D, Catley L, Li G, et al: A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell 8:407-419, 2005.
41. Hideshima T, Bradner JE, Wong J, et al: Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A 102:8567-8572, 2005.
42. Mitsiades CS, Mitsiades NS, McMullan CJ, et al: Transcriptional signature of histone deacetylase inhibition in multiple myeloma: Biological and clinical implications. Proc Natl Acad Sci U S A 101:540-545, 2004.
43. Richardson P, Schlossman R, Mitsiades C, et al: Phase I clinical trial of oral administration of the histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA) in patients with relapsed/refractory multiple myeloma (MM) (abstract 1503). Blood 104:420a, 2004.
44. Mitsiades CS, Mitsiades NS, McMullan CJ, et al: Antimyeloma activity of heat shock protein-90 inhibition. Blood 107:1092-1100, 2006.
45. Richardson P, Chanan-Khan A, Alsina M, et al: Safety and activity of KOS-953 in patients with relapsed refractory multiple myeloma (MM): Interim results of a phase 1 trial (abstract 361). Blood 106:109a, 2005.
46. Mitsiades CS, Mitsiades N, Rooney M, et al: IPI-504: A novel hsp90 inhibitor with in vitro and in vivo anti-tumor activity (abstract 2403). Blood 104(11):660a, 2004.
47. Chanan-Khan AA, Richardson PG, Alsina M, et al: Phase 1 clinical trial of KOS-953 + bortezomib (BZ) in relapsed refractory multiple myeloma (MM) (abstract 362). Blood 106:109a, 2005.
48. Hideshima T, Catley L, Yasui H, et al: Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 107:4053-4062, 2006.
49. Richardson P, Lonial S, Jakubowiak J, et al: A multicenter phase II study of perifosine (KRX-0401) alone and in combination with dexamethasone (Dex) for patients with relapsed or relapsed/refractory multiple myeloma (MM) (abstract 3582). Blood 108:1023a, 2006.
50. Mitsiades N, McMullan C, Poulaki V, et al: The mTOR Inhibitor RAD001 (everolimus) is active against multiple myeloma cells in vitro and in vivo (abstract 1496). Blood 104:418a, 2004.
51. Shi Y, Gera J, Hu L, et al: Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res 62:5027-5034, 2002.
52. Raje N, Kumar S, Hideshima T, et al: Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood 104:4188-4193, 2004.
53. Francis LK, Alsayed Y, Leleu X, et al: Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res 12:6826-6835, 2006.
54. Podar K, Raab MS, Zhang J, et al: Targeting PKC in multiple myeloma: In vitro and in vivo effects of the novel, orally available small-molecule inhibitor enzastaurin (LY317615.HCl). Blood 109:1669-1677, 2006.
55. Hu L, Shi Y, Hsu JH, et al: Downstream effectors of oncogenic ras in multiple myeloma cells. Blood 101:3126-3135, 2003.
56. Hideshima T, Podar K, Chauhan D, et al: p38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene 23:8766-8776, 2004.
57. Siegel D, Krishnan A, Lonial S, et al: Phase II trial of SCIO-469 as monotherapy (M) or in combination with bortezomib (MB) in relapsed refractory multiple myeloma (MM) (abstract 3580). Blood 108:1022a, 2006.
58. Mateos M, Blade J, Prosper F, et al: Preliminary report from an exploratory phase II trial with plitidepsin (Aplidin) in patients with refractory/relapsed multiple myeloma (abstract 2569). Blood 106, 2005.
59. Schey SA, Fields P, Bartlett JB, et al: Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol 22:3269-3276, 2004.
60. Mitsiades C, Benimetskaya L, Menon K, et al: Defibrotide (DF), an orally bioavailable modulator of myeloma tumor-microenvironment interactions: Molecular sequelae and clinical implications (abstract 3523). Blood 108:1005a, 2006.
61. Palumbo A, Rus C, Rossi D, et al: A multi-center phase I/II study of melphalan, prednisone, thalidomide and defibrotide in avanced multiple myeloma patients (abstract 3560). Blood 108:1016a, 2006.
62. Tai YT, Li X, Tong X, et al: Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer Res 65:5898-5906, 2005.
63. Hussein M, Niesvizky R, Munshi N, et al: A phase I, multi-dose, dose escalation study of SGN-40 (anti-huCD40 mAb) in patients with refractory or recurrent multiple myeloma (abstract 2413). Blood 104:663a, 2004.
64. Hussein M, Berenson J, Niesvizky R, et al: Results of a phase I trial of SGN-40 (anti-huCD40 mAb) in patients with relapsed multiple myeloma (abstract 3576). Blood 108:1021a, 2006.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.