Optimizing the Dose and Schedule of Darbepoetin Alfa in Patients With Chemotherapy-Induced Anemia
Chemotherapy-induced anemia is common in patients who have cancer. Erythropoiesis-stimulating proteins such as epoetin alfa (Procrit) and darbepoetin alfa (Aranesp) have been shown to improve hematologic and clinical outcomes in these patients. Darbepoetin alfa has a longer serum half-life than epoetin alfa, making less frequent administration possible and offering the possibility of synchronizing the administration of erythropoietic therapy and chemotherapy. Several clinical trials have evaluated the utility of darbepoetin alfa given every 3 weeks (q3wk) in patients with chemotherapy-induced anemia. An exploratory study showed that darbepoetin alfa q3wk stabilized hemoglobin levels and reduced transfusion requirements. It was also shown that giving darbepoetin alfa q3wk at the same time as the chemotherapy produced hematopoietic benefits similar to those observed when it is given later in the chemotherapy cycle. The q3wk dosing schedule was effective in patients with mild and moderate anemia, and treatment goals were achieved in most of them. The equivalence of q3wk and qwk darbepoetin alfa has also been established. Synchronous administration of darbepoetin alfa with chemotherapy is a convenient option for patients with chemotherapy-induced anemia, with clinical trials showing it to be an effective treatment strategy.
Chemotherapy-induced anemia is common in patients who have cancer. Erythropoiesis-stimulating proteins such as epoetin alfa (Procrit) and darbepoetin alfa (Aranesp) have been shown to improve hematologic and clinical outcomes in these patients. Darbepoetin alfa has a longer serum half-life than epoetin alfa, making less frequent administration possible and offering the possibility of synchronizing the administration of erythropoietic therapy and chemotherapy. Several clinical trials have evaluated the utility of darbepoetin alfa given every 3 weeks (q3wk) in patients with chemotherapy-induced anemia. An exploratory study showed that darbepoetin alfa q3wk stabilized hemoglobin levels and reduced transfusion requirements. It was also shown that giving darbepoetin alfa q3wk at the same time as the chemotherapy produced hematopoietic benefits similar to those observed when it is given later in the chemotherapy cycle. The q3wk dosing schedule was effective in patients with mild and moderate anemia, and treatment goals were achieved in most of them. The equivalence of q3wk and qwk darbepoetin alfa has also been established. Synchronous administration of darbepoetin alfa with chemotherapy is a convenient option for patients with chemotherapy-induced anemia, with clinical trials showing it to be an effective treatment strategy.
Debilitating anemia resulting from both the disease and its treatment is common in patients who have cancer.[1] Treating chemotherapy-induced anemia with erythropoiesis-stimulating proteins such as epoetin alfa (Epogen) and darbepoetin alfa (Aranesp) has been shown to increase hemoglobin (Hgb) levels, reduce the need for red blood cell transfusions, and improve quality of life.[2-13] Darbepoetin alfa has a greater sialic acid-containing carbohydrate content than epoetin alfa, which results in a longer serum half-life and greater potency.[14] This makes it possible to dose less frequently without any loss of efficacy. In particular, the ability to administer darbepoetin alfa every 3 weeks (q3wk) would provide a convenient therapeutic option for patients, caregivers, and health care providers, because most patients are treated with 3-week cycles of chemotherapy.
Of interest with the less-frequent dosing schedules of darbepoetin alfa is the effect of chemotherapy-induced myelosuppression on the efficacy of erythropoiesis stimulation, which could influence the timing of the administration of darbepoetin alfa. Phase II and III clinical trials have been conducted to evaluate the efficacy of darbepoetin alfa q3wk in a variety of settings and to determine the optimal timing of administration of darbepoetin alfa with chemotherapy.
Clinical Development of q3wk Dosing of Darbepoetin Alfa for Chemotherapy-Induced Anemia
A pilot randomized placebo-controlled dose-finding phase II study showed that darbepoetin alfa q3wk ameliorated anemia in patients with solid tumors.[3] Patients were eligible for the study if they were being treated with cyclic chemotherapy, had a life expectancy of at least 6 months, and had Hgb levels of 11 g/dL or less. They were randomized to darbepoetin alfa (at a dose of 4.5, 6.75, 9, 12, 13.5, or 15 µg/kg) or to placebo q3wk by subcutaneous (SC) injection. No dose increases for inadequate responses were permitted during the double-blind phase of the study, which lasted for a total of 12 weeks.
There was a dose response in terms of the percentage of patients with a Hgb response (defined as an increase in Hgb level of 2 g/dL or more in the absence of red blood cell transfusions in the previous 28 days) with darbepoetin alfa 4.5 µg/kg (24%) to 12 µg/kg (62%); no additional effects were seen with doses higher than 12 µg/kg. The response rate in the placebo group was 14%. There were hematopoietic responses (defined as a Hgb response or a Hgb level of 12 g/dL or higher in the absence of red blood cell transfusions in the previous 28 days) in more than half of patients treated with darbepoetin alfa 6.75 µg/kg (52%) and in 31% of those treated with placebo. There were red blood cell transfusions in fewer patients treated with darbepoetin alfa (19% to 30%) than with placebo (46%). The q3wk darbepoetin alfa schedule was well tolerated, with a safety profile comparable to that of placebo.
The results of this study indicate that q3wk dosing of darbepoetin alfa, at doses similar to those in more-frequent schedules, can increase Hgb to the target levels and reduce transfusion requirements.
The interplay between the effects of chemotherapy and erythropoiesis-stimulating proteins was explored in a randomized study that compared q3wk darbepoetin alfa given on a synchronous or asynchronous schedule relative to the chemotherapy in patients with nonmyeloid malignancies.[15] Patients treated with chemotherapy q3wk who had anemia (pretreatment Hgb level 9-11 g/dL) were randomized to darbepoetin alfa at a dosage of 6.75 µg/kg q3wk by SC injection on the same day as their chemotherapy (synchronous dosing) or on day 15 of the chemotherapy cycle (asynchronous dosing). To ensure that delays and modifications in the chemotherapy, which was given for up to 16 weeks, did not confound the study outcomes, changes in the Hgb levels were assessed at 6 weeks.
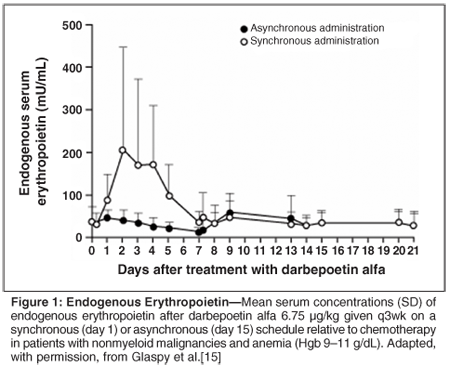
The serum levels of endogenous erythropoietin remained relatively constant during the chemotherapy cycle in the asynchronous group, but they increased by approximately fivefold in the synchronous group, peaking at day 2 and returning to near pretreatment levels by day 7 (Figure 1). Synchronous dosing was associated with a greater peak serum concentration of darbepoetin alfa and area under the concentration-time curve than asynchronous dosing, indicating greater exposure to the study drug in the synchronous group. The serum concentrations of darbepoetin alfa declined in the synchronous group in a monophasic manner after the peak concentration had been reached, but in the asynchronous group the decline in serum concentration was interrupted for approximately 3 days after the chemotherapy (given 1 week after the darbepoetin alfa) before declining again (Figure 2).
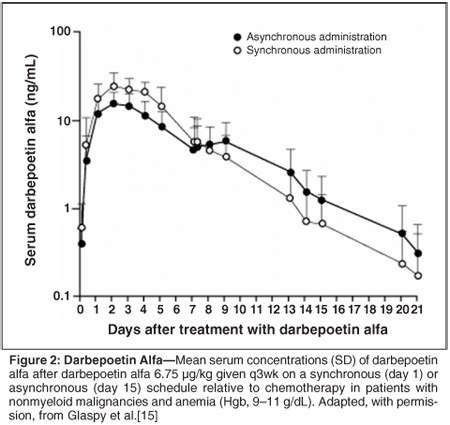
These findings suggest that the clearance of endogenous erythropoietin and darbepoetin alfa is interrupted by chemotherapy, possibly because it suppresses receptor-bearing cells in the bone marrow that contribute to clearance. No differences between the groups were seen in the magnitude or rate of the changes in Hgb levels. The levels stabilized or declined in the week immediately after the chemotherapy in both groups, and then rose in the remainder of the chemotherapy cycle. The rates of hematopoietic responses (a 2 g/dL or greater increase in Hgb level or an Hgb level of 12 g/dL or greater in the absence of red blood cell transfusions in the previous 28 days) at the end of the study were similar in the asynchronous (69%; 95% confidence level [CI] = 52%-86%) and synchronous (81%; 95% CI = 61%-100%) groups (Figure 3). These rates compare favorably with those with more-frequent administration of darbepoetin alfa at equivalent doses (60%-66% with darbepoetin alfa at 2.25 µg/kg/wk).[4,16]
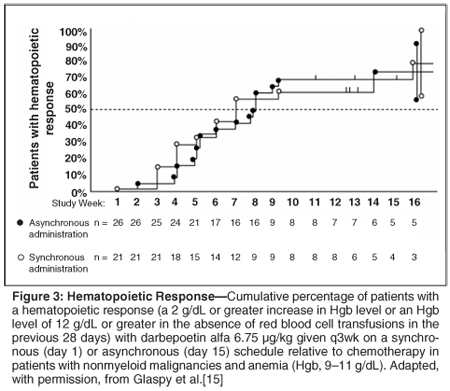
This study shows that synchronous and asynchronous dosing of darbepoetin alfa produce similar hematopoietic benefit, supporting the use of the more convenient synchronous schedule in clinical practice.
A fixed dose of darbepoetin alfa given once a week has been shown to be as effective as a weight-based weekly dose in patients with nonmyeloid malignancies and anemia.[17] The equivalence of fixed-dose darbepoetin alfa q3wk and weight-based once-weekly doses was evaluated in a large (N = 705) randomized double-blind European trial using a noninferiority design in patients treated with chemotherapy for nonmyeloid malignancies who had pretreatment Hgb levels of less than 11 g/dL.[18] Patients were randomized to darbepoetin alfa 2.25 µg/kg once a week by SC injection or to darbepoetin alfa
500 µg q3wk by SC injection. Dose modifications in accordance with the recommendations in the US prescribing information for darbepoetin alfa[19] were permitted.
Noninferiority would be achieved if the 95% CI of the difference in requirements for red blood cell transfusions between groups did not exceed 12.5%, a margin based on results from two previous placebo-controlled trials.[4,20] When adjusted for stratification factors, the difference in the proportion of patients in whom transfusions were required was 6.7% (95% CI = 0.2%-13.2%) in favor of q3wk dosing, demonstrating noninferiority between the dosages. Hemoglobin levels of 11 g/dL or higher were achieved in similar proportions of patients in both treatment groups, after adjusting for stratification factors (q3wk, 84%; qwk, 77%), and the Hgb profiles were almost identical in the groups, as were changes in their quality of life on the Functional Assessment of Cancer Therapy-Fatigue scale. There were no differences in adverse events, including cardiovascular or thrombotic adverse events, between the groups.
Summary
Giving erythropoiesis-stimulating proteins at the same time as chemotherapy is a convenient treatmentoption for patients who have chemotherapy-induced anemia, and it has been commonly done in clinical practice when possible. Until recently, however, there has been little understanding of the complex interaction between chemotherapy and erythropoiesis. The availability of longer-acting darbepoetin alfa, offering the possibility of dosing once per chemotherapy cycle, brought with it a greater need to evaluate these interactions. The hematopoietic and clinical responses with q3wk of darbepoetin alfa appear indistinguishable from those with qwk dosing. Furthermore, the hematopoietic effect of darbepoetin alfa is not compromised when it is given at the same time as the chemotherapy. The use of fixed rather than weight-based dosing offers further opportunity to simplify the scheduling and administration of erythropoietic therapy.
Disclosures:
Dr. Glaspy has received research support from Amgen and Ortho Biotech.
References:
1. Knight K, Wade S, Balducci L: Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am J Med 116(suppl 7A):11S-26S, 2004.
2. Schwartzberg LS, Yee LK, Senecal FM, et al: A randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 9:696-707, 2004.
3. Kotasek D, Steger G, Faught W, et al: Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy: Results of a double-blind, placebo-controlled, randomised study. Eur J Cancer 39:2026-2034, 2003.
4. Vansteenkiste J, Pirker R, Massuti B, et al: Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 94:1211-1220, 2002.
5. Glaspy J, Bukowski R, Steinberg D, et al: Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. Procrit Study Group. J Clin Oncol 15:1218-1234, 1997.
6. Demetri GD, Kris M, Wade J, et al: Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: Results from a prospective community oncology study. Procrit Study Group. J Clin Oncol 16:3412-3425, 1998.
7. Gabrilove JL, Cleeland CS, Livingston RB, et al: Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: Improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol 19:2875-2882, 2001.
8. Seidenfeld J, Piper M, Flamm C, et al: Epoetin treatment of anemia associated with cancer therapy: A systematic review and meta-analysis of controlled clinical trials. J Natl Cancer Inst 93:1204-1214, 2001.
9. Crawford J, Cella D, Cleeland CS, et al: Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 95:888-895, 2002.
10. Vadhan-Raj S, Mirtsching B, Charu V, et al: Assessment of hematologic effects and fatigue in cancer patients with chemotherapy-induced anemia given darbepoetin alfa every two weeks. J Support Oncol 1:131-138, 2003.
11. Patton J, Reeves T, Wallace J: Effectiveness of darbepoetin alfa versus epoetin alfa in patients with chemotherapy-induced anemia treated in clinical practice. Oncologist 9:451-458, 2004.
12. Vansteenkiste J, Tomita D, Rossi G, et al: Darbepoetin alfa in lung cancer patients on chemotherapy: A retrospective comparison of outcomes in patients with mild versus moderate-to-severe anaemia at baseline. Support Care Cancer 12:253-262, 2004.
13. Berndt E, Kallich J, McDermott A, et al: Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmacoeconomics 23:505-514, 2005.
14. Egrie JC, Browne JK: Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer 84(suppl 1):3-10, 2001.
15. Glaspy J, Henry D, Patel R, et al: Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: A randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. Eur JCancer 41:1140-1149, 2005.
16. Hedenus M, Hansen S, Taylor K, et al: Randomized, dose-finding study of darbepoetin alfa in anaemic patients with lympho-proliferative malignancies. Br J Haematol 119:79-86, 2002.
17. Hesketh PJ, Arena F, Patel D, et al: A randomized controlled trial of darbepoetin alfa administered as a fixed or weight-based dose using a front-loading schedule in patients with anemia who have nonmyeloid malignancies. Cancer 100:859-868, 2004.
18. Canon JL, Vansteenkiste J, Bodoky G, et al: Randomized, double-blind, active-controlled trial of every-3-week darbepoetin alfa for the treatment of chemotherapy-induced anemia. J Natl Cancer Inst 98:273-284, 2006.
19. Aranesp (darbepoetin alfa) prescribing information. Thousand Oaks, Calif, Amgen, 2005.
20. Hedenus M, Adriansson M, San Miguel J, et al: Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: A randomized, double-blind, placebo-controlled study. Br J Haematol 122:394-403, 2003.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.