(P081) An Analysis of Soft Tissue Anatomy and Tracking of the Prostate Using Transperineal Ultrasound
Transperineal ultrasound allows clinicians to both detect the anatomy of the pelvis in comparable detail with MRI and appreciate intrafraction motion of the prostate and nearby critical structures in order to deliver more precise radiotherapy.
P081: Figure
Vivek N. Patel, MD, Kyle Padgett, PhD, Elizabeth Bossart, PhD, Laura Freedman, MD, Adrian Ishkanian, MD, MSc, Alan Pollack, MD, PhD, Matthew Abramowitz, MD; University of Miami Miller School of Medicine
Purpose and Objectives: Transperineal ultrasound (TPUS) imaging is capable of providing low-cost, noninvasive, and accurate anatomic imaging of the male pelvis without the use of ionizing radiation. TPUS imaging can also be utilized during external beam radiation therapy (EBRT) to aid in treatment simulation to improve visualization of soft tissue anatomy. Furthermore, this modality may be used during daily treatment delivery to localize the prostate and organs at risk (OARs) within the pelvis using native anatomic structures. Thus, this procedure would allow clinicians to perform precise intrafraction prostate-tracking. Magnetic resonance imaging (MRI) is currently employed as an adjunct to CT for enhanced soft tissue imaging. However, this study requires image fusion with CT, which can be a source of uncertainty and can not be used during treatment. In contrast, TPUS is portable and functions via benign acoustic waves, which have not been shown to affect the treatment field. Apart from these inherent advantages, TPUS imaging is more cost-effective than MRI. Given these advantageous features, we demonstrate that TPUS can identify structures of the male pelvis similar to those images gathered from an MRI prior to simulation.
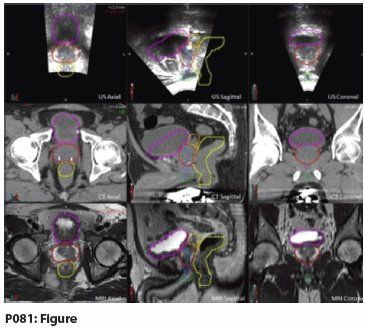
Materials and Methods: In order to evaluate the effectiveness of TPUS imaging, we analyzed the data collected for seven patients as part of an institutional review board (IRB)-approved clinical trial evaluating TPUS for prostate-tracking. These patients had pathologically confirmed prostate cancer and underwent definitive treatment with external radiation. All patients had a 3T MRI prior to CT simulation. During CT simulation, the patients had a 3D TPUS (Clarity system, Elekta). These images were subsequently transferred to the Clarity Automated Fusion and Contouring Workstation (V.3.0.0.109) and were fused. Thereafter, the fused images were transferred to MIM Maestro (V.6.1.7DB04-0B) for contouring. The MRI images for all patients were contoured for the prostate and OARs, including the bladder, rectum, seminal vesicles, penile bulb, and the genitourinary diaphragm. The MRI-contoured structures were subsequently overlaid on the CT and TPUS.
Results: When comparing the contoured images on the MRI and TPUS, it is apparent that both modalities are superior to CT and similarly capable of demarcating soft tissue anatomy, such as the penile bulb (green), bladder (magenta), urethra (blue), genitourinary diaphragm (purple), prostate (red), seminal vesicles (orange), and rectum (yellow). We have provided an example of an MRI-contoured structure set overlaid on CT and US to visually portray this observation. The advantage of the TPUS during simulation and treatment can be extrapolated from these contoured images. These images may also serve as an atlas to delineate structures that can be visualized during radiotherapy.
Conclusions: TPUS allows clinicians to both detect the anatomy of the pelvis in comparable detail with MRI and appreciate intrafraction motion of the prostate and nearby critical structures in order to deliver more precise radiotherapy.