(P091) The Clinical Significance of Overlap or Underlap of the Prescription Isodose Line and Prostate Contour in Brachytherapy for Low-Risk Prostate Adenocarcinoma
While biochemical progression-free survival (BPFS) for prostate cancer treated with brachytherapy is excellent, reported outcomes differ between groups. One hypothesis that has been proposed for improved biochemical outcomes is prescribing to a planning target volume (PTV) that extends substantially beyond the prostate. We tested this hypothesis by analyzing the effect of overlap vs underlap between prescription isodose lines and prostate contours on BPFS. We also assessed whether these spatial differences are correlated with increased acute toxicity.
Table P091
Jerry T. Liu, MD, Michael Buckstein, MD, PhD, Todd J. Carpenter, MD, Asher Mandel, Nelson N. Stone, MD, Richard G. Stock, MD; Icahn School of Medicine at Mount Sinai
Objective: While biochemical progression-free survival (BPFS) for prostate cancer treated with brachytherapy is excellent, reported outcomes differ between groups. One hypothesis that has been proposed for improved biochemical outcomes is prescribing to a planning target volume (PTV) that extends substantially beyond the prostate. We tested this hypothesis by analyzing the effect of overlap vs underlap between prescription isodose lines and prostate contours on BPFS. We also assessed whether these spatial differences are correlated with increased acute toxicity.
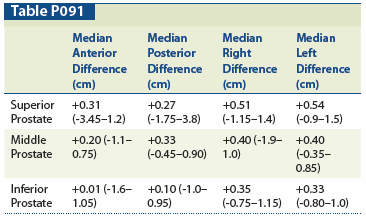
Methods: Patients with biopsy-proven prostate adenocarcinoma definitively treated with an 125I implant ± androgen deprivation therapy and minimum 3 years of follow-up were selected from our institutional database. Implants were prescribed, in general, to 160 Gy, and 30-day CT-based postimplant dosimetry was performed for all patients. For superior (1 cm below superior–most axial slice of prostate), middle (midpoint of superior-inferior extent), and inferior (1 cm above inferior–most axial slice) portions of the prostate, the difference between the 160-Gy isodose line and prostate contour was measured in four (anterior, posterior, right, and left) axial directions. The presence of overlap vs underlap at any of the anatomic points was analyzed for effects on BPFS, change in International Prostate Symptom Score (IPSS) (from pretreatment to first post-treatment 3-month follow-up score), urinary retention, hematuria, and proctitis. Toxicity ratings were based on Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancerg> (RTOG/EORTC) long-term toxicity scale and Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0). Proctitis events were confirmed endoscopically.
Results: With a median follow-up of 4.1 years, 546 patients met inclusion criteria. Actuarial 5-year BPFS was 97.5%. As summarized in the Table, there was a median overlap of the 160-Gy isodose line for all 12 anatomic locations. There were no significant differences in BPFS when comparing patients with overlap vs underlap at any site or cumulative axial direction (eg, all anterior sites). In addition, there were no significant differences in BPFS when comparing patients with overlaps at any site that were above vs below the median overlap for each respective site. With regard to toxicities, overlap vs underlap affected change in IPSS at the mid-left gland (P = .040, two-sided), proctitis incidence at the inferior-left gland (P = .003, two-sided), and incidence of retention at the inferior-left gland (P = .003, two-sided). Overlap vs underlap was not found to affect toxicities at any of the other sites.
Conclusion: While presence and degree of overlap of the prescription isodose line relative to the prostate gland did not appear to affect BPFS for low-risk patients, overlap at certain sites was found to be associated with increased GI and GU toxicity. These findings did not, however, have a clear anatomic correlate. Further study is needed with larger numbers in a higher-risk-group population to test the effect of overlap on BPFS and toxicity.