Panitumumab effective in normal KRAS subset
In unselected patients, results with the anti-epidermal growth factor receptor (EGFR) antibody panitumumab (Vectibix) in metastatic colorectal cancer patients were disappointing. However, patients with wild-type KRAS tumor status did benefit from panitumumab, according to several studies reported at the recent 2008 Gastrointestinal Cancers Symposium.

ORLANDO-In unselected patients, results with the anti-epidermal growth factor receptor (EGFR) antibody panitumumab (Vectibix) in metastatic colorectal cancer patients were disappointing. However, patients with wild-type KRAS tumor status did benefit from panitumumab, according to several studies reported at the recent 2008 Gastrointestinal Cancers Symposium.
Panitumumab was FDA approved in the United States in 2006 as monotherapy for the treatment of metastatic EGFR-expressing colorectal cancer after disease progression on or following fluorouracil (5-FU)-, oxaliplatin (Eloxatin)-, or irinotecan (Camptosar)-containing chemotherapy regimens, based on a phase III trial that showed it prolonged disease-free survival, compared with best supportive care (Van Cutsem et al: J Clin Oncol 25:1658-1664, 2007).
Investigators at the GI cancers meeting reported results for panitumumab as a single agent and in combination with other agents in the first- and second-line metastatic treatment settings.
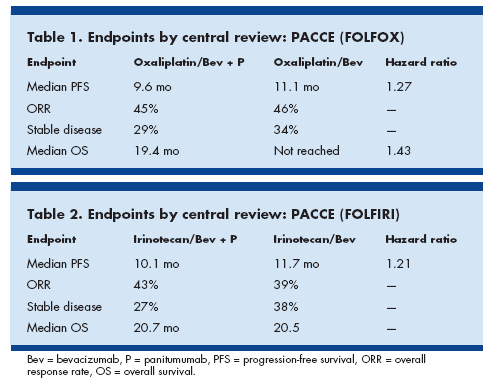
An interim analysis of the randomized open-label PACCE (Panitumumab Advanced Colorectal Cancer Evaluation) study showed that adding panitumumab to first-line chemotherapy-plus-bevacizumab (Avastin) regimens for metastatic disease offered no additional benefits in terms of progression-free or overall survival, while toxicity was increased.
In March 2007, the data monitoring committee recommended discontinuation of panitumumab dosing in the
PACCE study after the drug demonstrated no additional benefit but more toxicity.
PACCE evaluated bevacizumab plus standard chemotherapy-irinotecan based or oxaliplatin based-with or without panitumumab 6 mg/kg every 2 weeks until disease progression.
FOLFOX/panitumumab
Results of the oxaliplatin-based (FOLFOX) comparisons in 823 patients (abstract 273) were presented at the meeting by Edith Mitchell, MD, professor of medicine, Thomas Jefferson University.
At a median follow-up of 12.2 months, progression-free survival was shorter in the panitumumab arm, dose intensity was lower, and toxicity was increased, “indicating that this combination has an unfavorable benefit-to-risk profile in unselected patients with metastatic colorectal cancer,” Dr. Mitchell reported.
Progression-free survival was 19.4 months in the panitumumab arm but has not been reached in the control arm, for a 43% increased chance of progression with panitumumab (see Table 1).
Grade 3-4 adverse events were also higher with panitumumab, especially skin toxicity (36% vs 1%).
“This was expected, but we also saw more grade 3-4 diarrhea (24% vs 13%), infections (18% vs 10%), dehydration (17% vs 6%), hypokalemia (10% vs 4%), and other toxicities in this arm,” she said.
FOLFIRI/panitumumab
J. Randolph Hecht, MD, associate professor of hematology/oncology, UCLA School of Medicine, presented the data for the irinotecan-based chemotherapy (FOLFIRI, Douillard regimen) comparisons in 230 patients (abstract 279).
He reported that the response rates appeared higher in panitumumab-treated patients (due to higher responses associated with wild type KRAS). However, no significant differences were observed in progression-free survival or overall survival (see Table 2).
“However, most patients withdrew due to nonprogressive events (59% on the panitumumab arm and 71% on the standard arm), similar to the oxaliplatin chemotherapy cohort. This limits the utility of progression-free survival as a valid endpoint in this study,” Dr. Hecht commented.
By local review, overall response rates by KRAS status were 70% in patients with wild type KRAS receiving panitumumab, compared with 59% receiving standard therapy, for an odds ratio of 1.71. Patients with mutant KRAS had similar response rates to either regimen, 41% and 44%, respectively. This analysis was not powered for statistical significance and is descriptive only, he said.
Significance of KRAS
The significance of KRAS tumor status in patients treated with this anti-EGFR antibody is becoming clear.
In a retrospective KRAS analysis from the pivotal phase III trial in chemorefractory disease (panitumumab vs best supportive care) (abstract 278), median progression-free survival was almost doubled in patients with wild type KRAS, compared to those with KRAS mutations. Mutations were identified in 43% of the 427 patients tested for KRAS, reported Rafael Amado, MD, executive director of oncology therapeutics, Amgen, Inc, Thousand Oaks, California.
Panitumumab monotherapy produced responses in 17% of wild type patients, but no responses were observed in patients with the mutated gene; stable disease was observed in 34% and 12%, respectively.
Median survival was 12.3 weeks in wild type patients, compared with only 7.3 weeks for patients with the mutation.
Adverse events were similar overall, though grade 3-4 skin toxicity was more common in patients with wild type KRAS (25% vs 13%). This may be because these patients remained on treatment longer and received twice as much drug as those with mutated KRAS, he suggested.
“Based on these findings, we believe that KRAS genotyping of tumors should be strongly considered in patients with metastatic colorectal cancer being treated with panitumumab monotherapy,” Dr. Amado said.
A multicenter phase II trial of 203 patients with chemorefractory metastatic disease also showed the benefit of panitumumab was restricted to patients with wild type KRAS (abstract 343). Median progression-free survival was 15.0 weeks with wild type KRAS vs 7.1 weeks with mutated KRAS, Dr. Hecht reported.
“All responders had wild type KRAS, and even those with stable disease as best response had some tumor shrinkage,” he pointed out. “There were no responders among patients with mutant KRAS and very few even with stable disease.”
The KRAS finding has also been noted in studies of cetuximab (Erbitux), another anti-EGFR antibody. In the first-line setting, a 52-patient study by Tabernero and colleagues (abstract 435) showed median progression-free survival to be nearly doubled in wild type KRAS patients treated with cetuximab followed by FOLFIRI/cetuximab, compared to patients with KRAS mutations.
EGFR expression ‘meaningless’
The phase II study reported by Dr. Hecht found that low EGFR membrane expression is virtually meaningless in the prediction of response to panitumumab. In the cohort, 40% of patients were EGFR negative (< 1% EGFR+ cells); 55% were EGFR-low (1% to 9% EGFR+ cells); 3 had > 9% EGFR+ cells, and 8 were unevaluable. Similar response rates, progression-free survival, and overall survival were seen in patients with negative or low levels of EGFR staining, Dr. Hecht said.
“While EGFR expression is still in the drug’s labeling, it has no predictive value. Technically, if a patient were EGFR negative, he or she could be denied treatment by a third-party payer. Fortunately, most payers seem willing not to request this test,” Dr. Hecht said.