PARP Inhibitors in Breast Cancer: BRCA and Beyond
The aim of this article is to review the preclinical data and rationale for PARP inhibitor use in the aforementioned settings, as well as the current status of the clinical development of these agents in the treatment of breast cancer, along with future directions for research in this field.
DNA repair is essential for the survival of both normal and cancer cells. An elaborate set of signaling pathways detect single-strand and double-strand DNA breaks and mediate either DNA repair or apoptosis if the damage is too great to repair. Poly(adenosine diphosphate [ADP]–ribose) polymerases (PARPs) play a key role in the repair of base damage via the base excision repair pathway. Pharmacological inhibition of PARP induces cell death in tumors with mutations in certain DNA repair pathways-such as the BRCA pathways of double-strand break repair-and when combined with chemotherapies that cause DNA damage. PARP inhibitors are being investigated as a monotherapy for the treatment of patients with BRCA1/2 mutations; in the treatment of triple-negative breast cancer, because of its molecular similarities to BRCA1-mutated malignancies; and as a strategy to potentiate the DNA-damaging effects of chemotherapy and radiation. The aim of this article is to review the preclinical data and rationale for PARP inhibitor use in the aforementioned settings, as well as the current status of the clinical development of these agents in the treatment of breast cancer, along with future directions for research in this field. Trials have been identified via searches of PubMed, clinicaltrials.gov, and the Proceedings of the American Society of Clinical Oncology Annual Meeting and the San Antonio Breast Cancer Symposium.
DNA Repair and PARP Function
DNA repair is critical for the survival of cells. Estimations of the number of DNA damage events that occur on a daily basis are in the thousands. A number of DNA repair systems allow for repair and survival. While this is a desirable outcome for normal cells, DNA repair also allows cancer cells to survive the DNA injury posed by chemotherapy or radiation. Thus, there is a long-standing interest in impeding DNA repair as a potential strategy for enhancing the activity of chemotherapy and radiation in the treatment of cancer.
DNA damage results from a variety of exogenous and endogenous insults. Multiple types of DNA repair mechanisms exist; these include pathways that repair single-strand breaks and others that repair double-strand breaks. The pathways that are predominately involved in double-strand break repair are the nonhomologous end-joining and homologous recombination pathways.[1] Homologous recombination is a highly accurate mechanism that repairs double-strand breaks in the S and G2 phases of the cell cycle. Integral to the function of the homologous recombination pathway are BRCA1 and BRCA2. Loss of function of these proteins via inherited gene mutations results in faulty homologous recombination.[2] This is likely a key step in tumorigenesis in individuals with BRCA1/2 mutations who are predisposed to the development of breast, ovarian, and other cancers.
Base excision repair (BER) is the key pathway for the repair of damaged bases caused by endogenous DNA damage. Poly(adenosine diphosphate [ADP]–ribose) polymerases (PARPs) detect the single-strand breaks that are induced to remove damaged bases.[3,4] At least 17 members of the PARP family have been described to date, although PARP1 and PARP2 are the most relevant to BER.[5]. PARPs also have a number of other key functions, including a role in the epigenetic regulation of chromatin and control of cell division via interaction with centromeres.[5]
Synthetic lethality
FIGURE 1
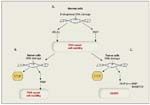
A normal cell with intact BRCA and PARP functions is able to repair DNA normally (A). In a tumor cell with a mutation in BRCA, intact PARP function results in ability to repair DNA and subsequent viability (B).
Central to the use of PARP inhibitors in the treatment of patients with malignancy related to BRCA1/2 mutations is the concept of synthetic lethality. Synthetic lethality refers to the situation in which two gene deficiencies that independently would not cause cell death are in fact lethal when they occur in combination.[6] In the setting of persons with BRCA mutations, the presence of the BRCA mutation and subsequent nonfunctional homologous recombination alone are not enough to cause tumor cell death. Applying the synthetic lethality concept, it was hypothesized that inhibiting an additional DNA repair pathway-namely BER-with a PARP inhibitor could cause the death of BRCA-deficient tumor cells (Figure 1). Specifically, the loss of PARP1 function results in the accumulation of single-strand DNA breaks, which are subsequently converted to double-strand breaks by cellular transcription and replication.[7] These double-strand breaks, which are typically repaired by homologous recombination or nonhomologous end-joining in normal cells, would accumulate in BRCA1- or BRCA2-deficient cells, leading to subsequent cell death.
This hypothesis was confirmed in two pivotal preclinical studies that demonstrated that loss of function of BRCA1 or BRCA2 conferred exquisite sensitivity to PARP inhibitors. Bryant et al observed that the PARP inhibitors NU1025 and AG14361 were profoundly cytotoxic in V-C8 (BRCA2-deficient) cells but did not affect V79 (BRCA2-expressing) cells. [8] Also, PARP inhibition affected survival of MCF7 (wild-type p53) and MDA-MB-231 (mutated p53) cells only when BRCA2 was depleted. In addition, the investigators also found significant response to AG14361 in xenograft tumor models of implanted BRCA2-deficient V-C8 cells.[8] Farmer et al described increased sensitivity to PARP inhibitors KU0058684 and KU0058948 of mouse embryonic stem cells lacking wild-type BRCA1 or BRCA2.[7] Of note, treatment with PARP inhibitors resulted in DNA damage, as indicated by the formation of gamma-H2AX foci, which occurs at sites of DNA damage in wild-type BRCA1- and BRCA2-deficient cells. However, repair of the DNA damage, as determined by measurement of nuclear RAD51 foci formation (which only occurs in the setting of BRCA-dependent homologous recombination), was only seen in the wild-type cells.[7]
Of interest, sensitivity to PARP inhibition has been observed in cells with defects in homologous recombination other than BRCA deficiency. These additional defects include phosphatase and tensin homolog (PTEN) deficiency,[9] ATM deficiency,[10,11] and Aurora A over-expression.[12] For instance, Mendes-Pereira et al recently suggested that the previously reported association between PTEN deficiency and genomic instability[13] is likely to result from defective homologous recombination. They found reduced activity of RAD51 and a reduced capacity to form nuclear RAD51 foci in response to DNA damage in PTEN-deficient colon and endometrial cancer cell lines.[9] They also found that PTEN deficiency correlates with a five-fold decrease in the number of double-strand breaks repaired by homologous recombination. Several PTEN-deficient tumor cell lines and xenograft models were found to have increased sensitivity to the PARP inhibitor olaparib.[9] These observations are of great interest, as they may broaden the population of patients who would potentially benefit from PARP inhibition, beyond the small population of patients with BRCA1/2 germline mutations.
Clinical Applications of PARP Inhibitors
BRCA1- and BRCA2-related breast cancer
TABLE 1

Current Status of the Clinical Development of PARP Inhibitors in the United States
Based on the above preclinical data on observed synthetic lethality in BRAC1/2-deficient cancers, a number of PARP inhibitors were developed for clinical use by various pharmaceutical companies (see Table 1-web site only). Fong et al reported the first phase I study using the oral PARP inhibitor olaparib (AZD2281; KU-0059436) [14]. The drug was initially given in a standard dose-escalating fashion to 60 patients with various malignancies, with an expansion subsequently performed at the recommended phase II dose in a population of BRCA1/2-deficient patients. The drug was generally well tolerated and the dose-limiting toxicities observed were reversible (grade 3 mood alterations and somnolence at the 400-mg twice-daily dose, and grade 4 thrombocytopenia and grade 3 somnolence at the 600-mg twice-daily dose). Adverse effects were not different in the BRCA mutation carriers enrolled in the study than in the non-carriers. The expansion cohort for patients with BRCA mutations consisted of 22 subjects with primarily breast, ovarian, or prostate cancers, who received olaparib at a dose of 200 mg twice daily. No objective tumor responses were seen in subjects without a BRCA mutation; however 12 out of 19 evaluable BRCA mutation carriers (63%) had a clinical benefit from treatment with olaparib. Nine of these patients (47%) had objective responses by Response Evaluation Criteria In Solid Tumors (RECIST) criteria, including a complete response in a patient with BRCA2-mutated breast cancer. Some patients had durable responses of over 1 year. Correlative pharmacodynamic studies demonstrated reduction in PAR levels and induction of gamma-H2AX foci indicative of double-strand breaks in tumor specimens and peripheral blood mononuclear cells.
Following the success observed in phase I, two phase II trials with olaparib-ICEBERG (International Collaborative Expertise for BRCA Education and Research through Genetics) 1 and 2-were carried out in breast and ovarian cancers, respectively. In the breast cancer study, presented by Tutt et al, 54 women with BRCA1- or BRCA2-deficient breast cancer were assigned to receive olaparib at either 400 mg (n = 24; cohort 1) or 100 mg (n = 24; cohort 2) twice daily in a nonrandomized, sequential fashion.[15] The 400-mg dose had been determined to be the maximum tolerated dose in the phase I study described above, while the 100-mg dose was shown to have clinical activity as well as pharmacodynamic activity without dose-limiting toxicity. BRCA mutation status was centrally determined for all patients. Eligibility requirements included treatment with at least one prior chemotherapy regimen, and the median number of prior therapies was three (range, one to five). Prior therapies included anthracycline and taxanes in the majority of patients; approximately a quarter of the patients had received platinum-containing therapies as well. The primary endpoint was objective response rate, which was 22% in the 100-mg twice-daily dose cohort and 41% at the 400-mg twice-daily dose. The clinical benefit rates were 26% and 52%, respectively. These response rates are quite remarkable for a biologically based therapy, particularly since they are similar to or better than the response rates expected with chemotherapy in anthracycline- and taxane-refractory patients. Responses were observed in heavily pretreated patients; the median response duration was 144 days at the 400-mg twice-daily dose and 141 days with the 100-mg twice-daily dose. There was no apparent difference in activity in patients with BRCA1 mutations and BRCA2 mutations, nor was activity related to the triple-negative status. As in the phase I study, olaparib was well tolerated and the most frequent adverse events at both dose levels were fatigue and nausea.
TABLE 2a

Clinical Trials With PARP Inhibitors in Breast CancerTABLE 2b

Clinical Trials With PARP Inhibitors in Breast Cancer (continued)
Although the authors recommended caution with interpretation of the improved response at the higher dose level, it was acknowledged that the lower dose appeared inferior in this trial as well as in the accompanying ovarian cancer trial.[16] In that phase II study, in heavily pretreated BRCA-mutated ovarian cancer patients, a response rate of 33% was observed at the 400-mg twice-daily dose, and a rate of 13% at the 100-mg twice-daily dose. It was suggested that the higher doses potentially had greater tissue penetration leading to enhanced target inhibition in tumors, despite the seemingly adequate pharmacodynamic activity that was observed in the surrogates of peripheral blood mononuclear cells and hair follicles at the lower dose. Unfortunately, serial tumor biopsies were not obtained to confirm these findings in the target tissue. Of particular note, there was a suggestion of diminished response to PARP inhibition in platinum-resistant patients, as there were few responses observed in that population in either the phase I or phase II studies. Small patient numbers limit any further exploration of this observation; however, it will be an important consideration in future trials, particularly since mechanisms of platinum resistance may be similar to mechanisms of resistance to PARP inhibitors. Exploration of these findings will require a clear and consistent definition of platinum resistance across trials. At this time, phase III testing of olaparib will take place in ovarian cancer rather than in breast cancer.[17]
There are currently a number of ongoing clinical studies in BRCA1/2 mutation carriers utilizing various PARP inhibitors both as single agents and in combination with chemotherapy. Early data with the PARP inhibitor MK-4827 have been presented that have also demonstrated single-agent activity in a population of BRCA-mutant breast cancer patients.[18] In that trial, half of the patients with BRCA1/2 mutations either had a response by RECIST criteria or had prolonged stable disease. Clinical trials with other PARP inhibitors given as a single agent are enrolling, and data are anticipated soon. In BRCA1/2-related malignancies, it is rational to expect greater activity with the combination of PARP inhibitors and chemotherapy than with PARP inhibitor monotherapy; however, there are no mature data from trials examining this question-particularly data that examine it in a randomized fashion. These ongoing studies will help to answer the subsequent questions that will arise regarding the most effective combinations, sequencing of therapy, and role of maintenance therapy. Table 2 summarizes the trials that have been reported evaluating PARP inhibitors in BRCA1/2-related breast cancer.
Sporadic breast cancer
In aggregate, the studies described above, in which significant activity of monotherapy is observed, have made a strong case for the use of PARP inhibitors in BRCA1/2-deficient tumors. Although BRCA mutations account for 70% to 85% of germline mutations in patients with heritable breast cancer, BRCA1/2-associated breast tumors represent only 5% to 10% of total breast cancers. The observation that some subtypes of sporadic breast cancer share many similarities with BRCA-mutated breast cancer have led to the clinical evaluation of PARP inhibitors in these tumors. The term “BRCAness” has been used to describe the similarities between basal-like or triple-negative breast cancer (TNBC) and BRCA1-mutated breast cancer.[19] In addition to being negative for the estrogen and progesterone receptors and HER2 expression by immunohistochemistry, both subtypes are frequently high-grade; demonstrate basal-like gene expression, mutated TP53, EGFR (epidermal growth factor receptor) expression, and an X chromosome inactivation pattern; and show an apparent sensitivity to platinum chemotherapies.[20]
Although spontaneous BRCA mutations are rare events, reduced expression of BRCA1 has been demonstrated in sporadic breast cancers. Multiple potential mechanisms have been described, including allelic loss of 17q (which houses BRCA1)[21]; hypermethylation of the BRCA1 regulatory region[22]; and higher levels of the BRCA1-negative regulator, ID4.[23] If these mechanisms result in nonfunctional homologous recombination, then there is the potential for PARP inhibitor monotherapy activity in TNBC. The possibility that TNBCs have abnormal DNA repair pathways has been evaluated by several groups. Lips et al assessed 163 TNBC samples for homologous recombination deficiency; they found that over half of the samples had a pattern of array comparative genomic hybridization (aCGH) that was BRCA1-like, that a quarter had BRCA promoter hypermethylation, and that a third had reduced BRCA1 mRNA expression. These defects, however, were not associated with response to neoadjuvant chemotherapy. Of interest, a BRCA2-like aCGH pattern was identified in 40% of estrogen receptor (ER)-positive tumors that did predict neoadjuvant chemotherapy response.[24] Similarly, Rodriguez et al have developed a defective DNA repair signature. This signature has been shown to predict anthracycline sensitivity and taxane resistance that is similar to the resistance seen in a BRCA1-mutated breast cancer in a sample of TNBC.[25] These and other assays to assess intact DNA repair function in TNBC will need to be evaluated in the setting of PARP inhibitor clinical trials to determine whether, in fact, there are defects in homologous recombination or other pathways that may be predictive of sensitivity to these agents.
The possibility that levels of PARP expression may play a role in chemotherapy sensitivity has been examined in breast cancers. Retrospective data sets showed that high levels of PARP are observed in some breast cancers. The German Breast Group evaluated specimens from the neoadjuvant GeparTrio trial. It was found that high levels of cytoplasmic PARP, but not nuclear PARP levels, correlated with a more aggressive phenotype with an unfavorable long-term prognosis, despite improved rates of response to neoadjuvant anthracycline- and taxane-based chemotherapy. These levels were highest in TNBC, although they were also demonstrated in hormone receptor–positive and HER2-positive tumors.[26] Another report by Domagala et al similarly examined nuclear and cytoplasmic PARP levels. The authors found that the majority of BRCA1-associated and non–BRCA1-related breast cancers expressed high levels of nuclear PARP. The assessment of cytoplasmic PARP revealed that, while expression of cytoplasmic PARP was rarer than nuclear PARP expression, BRCA1-associated cancers had twice the frequency of cytoplasmic PARP expression compared with non–BRCA-related cancers. As in the GeparTrio data, cytoplasmic levels of PARP were associated with high-grade tumors. PARP levels were not detected in a fraction of both TNBC and BRCA-related breast cancers. The authors speculated that these tumors could be insensitive to PARP inhibitors, although this was not evaluated in patient samples in the setting of PARP inhibitor treatment.[27] These observations suggest that the activity of PARP inhibitors in sporadic tumors may be due to the other functions of PARPs beyond DNA repair, since cytoplasmic PARP was as well correlated with aggressiveness and response as was nuclear PARP, which is actually located at the site of DNA repair. The levels of PARP in a given tumor could be postulated to contribute to PARP inhibitor sensitivity, although this has yet to be confirmed in prospective studies.
PARP inhibitor monotherapy in sporadic TNBC has been evaluated in a small study. Gelmon et al reported a phase II study with olaparib in four cohorts of patients.[28] These cohorts included BRCA-negative/unknown ovarian cancer, BRCA-negative/unknown TNBC, BRCA-positive ovarian cancer, and BRCA-positive breast cancer. Activity was seen in all arms with the exception of the sporadic TNBC cohort. No single-agent activity with olaparib was seen in that group of 15 patients, leading to early closure of that arm. Of note, objective responses by RECIST criteria were only observed in ovarian cancer patients, including those in the BRCA-negative/unknown ovarian cancer arm; these findings have prompted a recent phase II trial in this group of patients, with continued activity observed.[29] There is an ongoing phase I study of veliparib (ABT-888) as a single agent in non–BRCA-mutated TNBC, the results of which are anticipated.[30] It may be too early to say that PARP inhibitor monotherapy is ineffective in sporadic TNBC. However, it appears that single-agent activity would require a loss of function of BRCA or other homologous recombination pathway members-a possibility that is under active study as described above but that has yet to be consistently demonstrated in TNBC. It would be of great benefit to identify a subset of TNBC with defective homologous recombination or PARP overexpression that would predict response to PARP inhibitors. This will require careful correlative analysis of TNBC patients participating in PARP inhibitor trials.
Iniparib and triple-negative breast cancer
Iniparib (BSI-201) was the first PARP inhibitor evaluated as a therapeutic strategy specifically for sporadic TNBC. Iniparib was initially believed to be an irreversible inhibitor of PARP1 and demonstrated effects consistent with PARP inhibition in preclinical studies. The phase I study with this agent was conducted in 23 patients with solid tumors; patients were escalated through seven doses levels up to 8 mg/kg, and dose-limiting toxicity was not reached.[31] Stable disease greater than 2 months was seen in 6 patients; BRCA status was not reported. A phase IB study was carried out in which iniparib was combined with various chemotherapeutic agents, including gemcitabine and carboplatin; tolerability and responses were demonstrated in a variety of tumor types.[32]
Subsequently, a phase II study was performed to explore the activity of iniparib in combination with chemotherapy in a population of patients with sporadic TNBC. O'Shaughnessy et al randomly assigned 123 patients to receive gemcitabine (Gemzar) (1000 mg/m2 × body surface area [BSA]) and carboplatin (area under the curve [AUC] = 2) on days 1 and 8, with or without iniparib (a at dose of 5.6 mg/kg given twice weekly).[33] Histologic evidence of ER/progesterone receptor (PR)–negativity and HER2-negativity was required, but central review of tissue was not required. Eligible patients could have had up to two prior chemotherapy regimens in the metastatic setting, although prior gemcitabine, platinum, or PARP inhibitor therapy was not allowed. More than half of the enrolled patients had three or more sites of metastatic disease, and approximately 40% had liver metastases. The primary endpoint was clinical benefit rate, defined as objective complete and partial responses and stable disease > 6 months. A significant improvement in all outcomes was noted with the addition of iniparib to gemcitabine/carboplatin. The clinical benefit rate was significantly improved-from 34% to 56%-with the addition of iniparib to chemotherapy. Progression-free survival (PFS) was prolonged from 3.6 months to 5.9 months (hazard ratio, 0.59; P = .01), and increased overall survival (OS)-from 7.7 months to 12.3 months (hazard ratio, 0.57; P = .01)-was observed as well. Importantly, there were no apparent significant differences in toxicity. Half of the patients initially randomized to receive chemotherapy alone crossed over to the iniparib arm, and minimal activity was observed after crossover. This result was felt to be similar to the low rate of single-agent olaparib activity in platinum-pretreated BRCA1/2 mutation–related breast cancer patients and may suggest similar mechanisms of resistance. Based on these encouraging results, accrual for a phase III study was rapidly completed.
The confirmatory phase III study was presented at the 2011 annual meeting of the American Society of Clinical Oncology.[34] A total of 519 patients were randomly assigned to receive gemcitabine and carboplatin alone or in combination with iniparib. As in the phase II trial, patients could have received 0 to 2 prior treatments for metastatic breast cancer. Sixty percent of enrolled patients had three or more sites of metastatic disease, and 60% had liver metastases. There was some imbalance in the disease-free interval in the first-line patients, with a disease-free interval of 15.9 months in the gemcitabine/carboplatin arm compared with an interval of 9.5 months in the gemcitabine/carboplatin/iniparib arm. Similar to the phase II trial, treatment was well tolerated in both arms. Despite the exciting phase II results, the phase III trial did not achieve statistical significance for the co–primary endpoints of PFS and OS. The prespecified type 1 error adjustment was a total alpha of 0.05, with significance for PFS set at 0.01 and for OS at 0.04. The reported median OS was 11.1 months in the chemotherapy-alone arm and 11.8 months when iniparib was added (P = .28); PFS was 4.1 months without iniparib and 5.1 months with the addition of iniparib (P = .027). Of interest, multivariate analysis was performed comparing first-line patients to second- and third-line patients. Although the hazard ratio for PFS was 0.88 (P = .37) in the first-line patients, in the second- and third-line patients, the hazard ratio for PFS was 0.67 (P = .011).When adjustment was made for the differences in the disease-free interval and a number of prespecifed factors (age, disease burden, Eastern Cooperative Oncology Group [ECOG] performance status, line of therapy, race, time since diagnosis of metastatic disease, visceral disease, and elevated alkaline phosphatase level), the hazard ratio for PFS in the intent-to-treat population was 0.74 (P = .004). After adjusting for the above factors, the hazard ratio for OS was 0.78 (P = .05).
TABLE 3

Comparison of Phase II and Phase III Results from the Gemcitabine/Carboplatin/Iniparib Trials
Compared with the phase II study, far more patients in the phase III study crossed over to the chemotherapy-with-iniparib arm after receiving chemotherapy alone-96% in the phase III study vs 51% in the phase II study. Trial eligibility was based on local assessment of ER/PR/HER2 status, and although tumor blocks on all patients were collected for central review, those results were not presented. Affymetrix gene profiling of 304 patient samples demonstrated much heterogeneity in this TNBC population. Although 55% of the samples were either basal-like, claudin-low, or normal breast–like, 30% were classified as ERBB2, and 20% were luminal B. Analysis of the DNA repair signature is planned. The results of the phase II and phase III gemcitabine/carboplatin/iniparib studies are summarized in Table 3.
Several factors might account for these discrepant and disappointing results. The imbalance in patient characteristics in the two study arms is important. If the control arm was comprised of patients with less aggressive disease, as reflected by a longer disease-free interval, then this group could be less refractory to chemotherapy and more likely to respond to control chemotherapy. Similarly, a less aggressive phenotype could be suggested by the fact that the second- and third-line patients showed benefit, whereas first-line patients did not. This might reflect the fact that the first-line patients-who had a poorer prognosis-progressed and died rapidly regardless of therapy arm. It also appears that the gemcitabine/carboplatin arm performed better with regard to the endpoint of OS in the phase III trial than it did in the phase II trial, although other endpoints were similar. In contrast, the results for PFS and OS in the iniparib arms appeared to be consistent between phase II and phase III, although higher response rates were seen in phase II. An additional confounding factor was the high crossover rate in the phase III trial, which was considerably higher than that in the phase II trial. While crossover could definitely affect PFS and OS, it should not affect response and clinical benefit rates, which would reflect responses prior to crossover. Both objective response rate and clinical benefit were not significantly different with either chemotherapy alone or the addition of iniparib in the phase III trial. Also, the crossover patients in the phase II study did not benefit from the addition of iniparib, but this information was not presented for the phase III trial. One could postulate that the iniparib story in TNBC might be similar to that of bevacizumab (Avastin) in breast cancer, reflecting the difficulty in improving OS in a population of patients who subsequently receive multiple lines of additional therapy. However, unlike with bevacizumab, there was no benefit in other endpoints (objective response rate, clinical benefit rate, and PFS) with the addition of iniparib to chemotherapy.
From a preclinical standpoint, it has been demonstrated that not all basal-like cancers lack ER, PR and HER2 (as determined by gene expression profiling); conversely, not all triple-negative breast cancers show a basal-like phenotype by expression array analysis.[35] Since there is incomplete phenotypic overlap between TNBC, basal, and BRCA1/2-mutated cancers, it is possible that a subset of TNBC, such as the claudin-low or normal breast–like tumors, might not harbor defects in the homologous recombination DNA repair system and therefore would not be affected to as great a degree by the combination of PARP inhibition and chemotherapy. Moreover, the reduced BRCA expression described in TNBC may be variable and heterogeneous, as opposed to the complete absence found in BRCA1/BRCA2-mutant tumors; if this is the case, the addition of a PARP inhibitor would not enhance activity beyond that seen with chemotherapy alone. In a population of TNBC patients, in which some patients would be BRCA1/2-positive, there is also the potential for disparate numbers of mutation carriers in the phase II and phase III studies, which could affect the results. Data on BRCA mutational status have been collected but not yet analyzed or reported. Preliminary correlative data from the phase III trial demonstrated great diversity in molecular subtypes enrolled, including subtypes of typically ER-positive tumors in which “BRCA-ness” would not be expected. This biological heterogeneity could also contribute to the discrepant results between the phase II and phase III trials.
Lastly, the mechanism of action of iniparib is not well understood. Recent data from Ji et al showed that there are clear differences between the mechanism of action of iniparib and those of other PARP inhibitors. These studies demonstrated that iniparib did not inhibit PARP1/2, whereas olaparib and veliparib did. Iniparib appeared to exert an effect on the telomerase pathway and on PARP5/6.[36] Additional recent data also suggest a mechanism of action for iniparib different from those of other PARP inhibitors.[37,38] Indeed, it was acknowledged by the phase III study investigators that iniparib does not inhibit PARP1/2 at physiologic drug concentrations, and the actual drug target(s) of iniparib have yet to be identified. This has a direct impact on the interpretation of clinical trials with iniparib. If the drug does not mechanistically inhibit PARP1/2, then its impact on homologous recombination–deficient tumors or TNBC may be irrelevant. A number of ongoing studies of iniparib should help to elucidate the mechanism of action and activity of this compound, as well as the best population for its use.
Other combinations of PARP inhibitors and chemotherapy
Although much of the research into clinical applications of PARP inhibitors has focused on tumors with known or presumed defects in homologous recombination, there is a large body of evidence about combining chemotherapies that induce DNA damage with PARP inhibitors as a mechanism for enhancing activity. Both preclinically and clinically, PARP inhibitors have been studied in combination with a number of DNA-damaging and other chemotherapeutic agents, including platinum salts,[39,40] temozolomide (Temodar; TMZ),[41,42] irinotecan (Camptosar),[39] taxanes,[43] doxorubicin and cyclophosphamide in combination,[44] and cyclophosphamide as a single agent (both as a bolus and metronomically dosed).[45,46] A number of clinical trials of various PARP inhibitors in combination with chemotherapy have been presented, predominately in abstract format, as summarized in Table 2.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
AG14361
AG014699/PF-01367338
Bevacizumab (Avastin)
BMN-673
Carboplatin
Cediranib
CEP-9722
Cyclophosphamide
Doxorubicin
Gemcitabine (Gemzar)
GP121016
Iniparib
INO-1001
Irinotecan (Camptosar)
KU0058684
KU0058948
MK-4827
NU1025
Olaparib (AZD2281)
Paclitaxel
Temozolomide (Temodar)
Veliparib
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Of particular interest is the observation of clinical activity with a chemotherapeutic agent not previously shown to have efficacy in breast cancer. Isakoff et al investigated the combination of veliparib and TMZ in metastatic breast cancer, based on the synergy between these two agents seen in breast cancer xenograft models. TMZ has minimal activity in breast cancer, perhaps because of robust repair of methylated DNA adducts by the BER pathway and O6-methylguanine DNA methyltransferase (MGMT). The investigators proposed that adding a PARP inhibitor to TMZ would prevent the repair of those methylated DNA adducts, leading to activity of the combination in metastatic breast cancer.[47] In their single-arm phase II trial of veliparib and TMZ, they recruited 41 patients to receive veliparib (40 mg PO BID, on days 1 through 7) and TMZ (150 mg/m2 PO QD, on days 1 through 5) on a 28-day cycle. Of the 41 patients recruited, 8 were carriers for BRCA1/2 mutations, 8 had a normal BRCA1/2 status, and 25 had an unknown BRCA1/2 status. Activity of the combination was limited to BRCA mutation carriers. In BRCA1/2 mutation carriers, 37.5% achieved an objective response, with a clinical benefit rate of 62.5%. These results are intriguing, and results of trials of the single-agent activity of veliparib as a comparator are awaited.
Future Directions
The story of PARP inhibitors, while still in the early stages, illustrates the bench-to-bedside potential of personalized cancer medicine. Many unanswered questions about the clinical application of PARP inhibitors remain. While single-agent activity has been clearly demonstrated in BRCA mutation carriers for olaparib in the phase II setting, data on the single-agent activity of other PARP inhibitors is awaited, as are the results of a large, confirmatory phase III trial. The best use of these agents in patients with BRCA1/2 mutations needs to be determined. Whether “induction” with chemotherapy and a PARP inhibitor and then “maintenance” with a single-agent PARP inhibitor is appropriate- or whether there exists a subset of patients who can avoid chemotherapy entirely and be treated with PARP inhibitors alone-remains to be seen. Whether PARP inhibitors might play a role in cancer prevention-ie, as a means of avoiding prophylactic surgeries in BRCA mutation carriers-is an attractive hypothesis, given the activity and apparent lack of toxicity of these agents in the available clinical treatment studies; however, the proposition remains highly speculative. The absence of data on long-term use of these agents and the theoretical concern that PARP inhibition could be genotoxic and might increase the risk of development of other cancers must also be addressed.[48]
It is a challenge to identify the patients who will benefit from PARP inhibition, especially in the sporadic TNBC setting, given the incomplete overlap between the genotypic and phenotypic profiles of BRCA1/2-mutated tumors and TNBC tumors. As clearly illustrated with iniparib, preclinical testing and confirmation of mechanism of action in correlative studies are crucial. In trials of PARP inhibitors in sporadic tumors, it is critical to develop a method for measuring the competency of homologous recombination and to determine other mechanisms of PARP function that may influence activity. Since the majority of clinical trials, particularly in sporadic breast cancer, have only been reported in abstract format, a more detailed presentation of the complete data is awaited. The apparent differences between the activity of PARP inhibitors in sporadic ovarian cancer and their activity in sporadic breast cancer- despite the similar efficacy of these agents in all BRCA1/2-mutated populations -are intriguing: they suggest a major difference in tumorigenesis between these two histologies in sporadic cases. Because these cancers were initially treated together in the same phase I studies, the varying responses in phase II are of interest. Exploration of the mechanisms of resistance and variable responses in sporadic breast and serous ovarian cancer will aid in the identification of the most appropriate populations in which to use PARP inhibitors.
As data accumulate on the efficacy of PARP inhibitors, determination of the mechanisms of resistance in nonresponders will be needed. It has been shown that a second mutation in the BRCA2 gene could “restore” the functionality lost with the initial inherited mutation.[49] Such a mutation could turn a BRCA-deficient cell into a “wild-type” one with competent homologous recombination, thereby conferring resistance to PARP inhibition. Other proposed mechanisms of resistance to PARP inhibitors, including upregulation of the gene encoding for the P-glycoprotein efflux pump and up-regulation of proteins that compete with the homologous recombination repair machinery (such as 53BP1[50]), still need further validation. Emerging data suggest that the effects of PARP inhibition in homologous recombination–deficient populations may not be exclusively due to abrogation of the BER pathway via synthetic lethality.[51] Patel et al hypothesized that if the effects of PARP inhibition on BRCA-mutated cells results from BER inactivation, then disabling other key proteins in the BER repair system should also kill homologous recombination–deficient cells. While they found, as expected, that PARP1 depletion killed BRCA2-deficient ovarian cancer cells, depletion of another key base excision repair protein (XRCC1) failed to kill the same BRCA2-deficient cells. They then went on to show that nonhomologous end-joining, an error-prone repair pathway that is ordinarily suppressed by PARP1, is preferentially activated in homologous recombination–deficient cells treated with PARP inhibitors. Moreover, knockdown or deletion of nonhomologous end-joining components prevented the cytotoxicity of PARP inhibitors and PARP1 depletion in homologous recombination–deficient cells, suggesting that PARP inhibitors kill homologous recombination–deficient cells by de-repressing the error-prone nonhomologous end-joining pathway.
Despite the challenges in defining the best application of PARP inhibitors, this class of agents has demonstrated great promise. PARP inhibitors will likely add substantially to at least the treatment of patients with heritable breast and ovarian cancer, if not to a larger population of cancer patients. Ongoing studies will have the challenge of determining the precise mechanisms of action, the most efficacious and tolerable chemotherapy combinations, and the mechanisms of resistance in both BRCA1/2-related cancers and sporadic cancers.
Financial Disclosure:This work was supported by the National Cancer Institute (U01 CA099168-07S2, P50 CA088843-10) and the Frieda G. and Saul F. Shapira BRCA Cancer Research Program, whose aims include conducting clinical trials of veliparib in BRCA-associated and sporadic cancers. The authors have no other significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411366-74
2. Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171-82.
3. Masson M, Niedergang C, Schreiber V, et al. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563-71.
4. El-Khamisy SF, Masutani M, Suzuki H, et al. A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acid Res. 2003;31:5526-33
5. Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 2008;13:3046-82.
6. Dobzhansky T. Genetics of natural populations; recombination and variability of Drosophila pseudoobscura. Genetics. 1946; 31:269-90.
7. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-21.
8. Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumors with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-7.
9. Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315-22.
10. Williamson CT, Muzik H, Turhan AG, et al. ATM deficiency sensitizes mantle cell lymphoma cells to poly (ADP-ribose) polymerase-1 inhibitors. Mol Cancer Ther. 2010; 9:347-57.
11. Weston VJ, Oldreive CE, Skowronska A, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116:4578-87.
12. Sourisseau T, Maniotis D, McCarthy A, et al. Aurora-A expressing tumour cells are deficient for homology-directed DNA double strand-break repair and sensitive to PARP inhibition. EMBO Mol Med. 2010:130-42.
13. Shen WH, Balajee AS, Wang J, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157-70.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009; 361:123-34.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-44.
16. Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245-51.
17. AstraZeneca press release. Available from: http://www.astrazeneca-us.com/search/?itemID=10810643. Accessed Aug 03 2011.
18. Schelman WR, Sandhu SK, Moreno Garcia V, et al. First-in-human trial of a poly(ADP)-ribose polymerase (PARP) inhibitor MK-4827 in advanced cancer patients with antitumor activity in BRCA-deficient tumors and sporadic ovarian cancers (soc). J Clin Oncol. 2011;29 (suppl; abstr 3102).
19. Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer. 2004;4:814-19.
20. Isakoff SJ. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 2010;16:53-61.
21. Futreal PA, Söderkvist P, Marks JR, et al. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992;52:2624-7.
22. Esteller M, Silva JM, Dominguez G, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Nat Can Inst. 2000; 92:564-9.
23. Umetani N, Mori T, Koyanagi K, et al. Aberrant hypermethylation of ID4 gene promoter region increases risk of lymph node metastasis in T1 breast cancer. Oncogene. 2005;24:4721-7.
24. Lips EH, Mulder L Hannemann J, et al. Indicators of homologous recombination deficiency in breast cancer and association with response to neoadjuvant chemotherapy. Ann Oncol. 2011;22:870-6.
25. Rodriguez AA, Makris A, Wu MF et al. DNA repair signature is associated with anthracycline response in triple negative breast cancer patients. Br Ca Res Treat. 2010;123;189-96.
26. von Minkwitz G, Müller BM, Loibl S, et al. Cytoplasmic poly(adenosine diphosphate–ribose) polymerase expression is predictive and prognostic in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011; 29:2150-7.
27. Domagala P, Huzarski T, Lubinski J, et al. PARP-1 expression in breast cancer including BRCA1-associated, triple negative and basal-like tumors: possible implications for PARP-1 inhibitor therapy. Breast Cancer Res Treat. 2011;127:861-9.
28. Gelmon KA, Hirte HW, Robidoux A, et al. Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer. J Clin Oncol.2010; 28:15s (suppl; abstr 3002).
29. Ledermann JA, Harter P, Gourley C, et al. Phase II randomized placebo-controlled study of olaparib (AZD2281) in patients with platinum-sensitive relapsed serous ovarian cancer (PSR SOC) J Clin Oncol. 2011;29(suppl; abstr 5003).
30. ABT-888 in treating patients with malignant solid tumors that did not respond to previous therapy. NCT00892736. Available from: http://clinicaltrials.gov/ct2/show/NCT00892736?term=NCT00892736&rank=1. Accessed on Aug 24 2011.
31. Kopetz S, Mita MM, Mok I, et al. First in human phase I study of BSI-201, a small molecule inhibitor of poly ADP-ribose polymerase (PARP) in subjects with advanced solid tumors. J Clin Oncol. 2008;26(May 20 suppl; abstr 3577).
32. Mahany JJ, Lewis N, Heath EI, et al. A phase IB study evaluating BSI-201 in combination with chemotherapy in subjects with advanced solid tumors. J Clin Oncol. 2008;26 (May 20 suppl; abstr 3579).
33. O'Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205-14.
34. O'Shaughnessy J, Schwartzberg LS, Danso MA, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin in metastatic triple-negative breast cancer. J Clin Oncol. 2011;29 (suppl; abstr 1007).
35. Badve S, Dabbs DJ, Schnitt SJ, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24:157-67.
36. Ji J, Lee MP, Kadota M, et al Pharmacodynamic and pathway analysis of three presumed inhibitors of poly (ADP-ribose) polymerase: ABT-888, AZD 2281, and BSI201. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 Apr 2-6; Orlando, Fla. AACR. 2011. Abstract nr 4527).
37. Maegley KA, Bingham P, Tatlock JH, et al. All PARP inhibitors are not equal: an in vitro mechanistic comparison of PF-01367338 to iniparib. J Clin Oncol. 2011;29 (suppl; abstr e13576).
38. Nagourney RA, Kenyon KR, Francisco FR, et al. Functional analysis of PARP inhibitors AZD 2281 and BSI-201 in human tumor primary cultures: a comparison of activity and examination of synergy with cytotoxic drugs. J Clin Oncol. 2011;29 (suppl; abstr e13599).
39. Evers B, Drost R, Schut E, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;
14:3916 -25.
40. Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105:17079-84.
41. Thomas HD, Calabrese CR, Batey MA, et al. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther. 2007;6:945-56.
42. Donawho CK, Luo Y, Penning TD, et al. ABT-888, an orally active poly(ADPribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728-37.
43. Dent RA, Lindeman GJ, Clemons M, et al. Safety and efficacy of the oral PARP inhibitor olaparib (AZD2281) in combination with paclitaxel for the first- or second-line treatment of patients with metastatic triple-negative breast cancer: results from the safety cohort of a phase I/II multicenter trial. J Clin Oncol. 2010;28:15s (suppl; abstr 1018).
44. Tan AR, Toppmeyer D, Stein MN, et al. Phase I trial of veliparib, (ABT-888), a poly(ADP-ribose) polymerase (PARP) inhibitor, in combination with doxorubicin and cyclophosphamide in breast cancer and other solid tumors. J Clin Oncol. 2011;29 (suppl; abstr 3041).
45. Kummar S, Chen AP, Ji JJ, et al. A phase I study of ABT-888 (A) in combination with metronomic cyclophosphamide (C) in adults with refractory solid tumors and lymphomas. J Clin Oncol. 2010;28:15s (suppl; abstr 2605).
46. Tan AR, Gibbon D, Stein MN. Preliminary results of a phase I trial of ABT-888, a poly(ADP-ribose) polymerase (PARP) inhibitor, in combination with cyclophosphamide. J Clin Oncol. 2010;28:15s (suppl; abstr 3000).
47. Isakoff SJ, Overmoyer B, Tung NM, et al. A phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancer. J Clin Oncol. 2010;28:15s (suppl; abstr 1019).
48. Calvert H, Azzariti A. The clinical development of inhibitors of poly(ADP-ribose) polymerase. Ann Oncol. 2011;22(Suppl ):i53-9.
49. Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111-5.
50. Bunting SF, Callen E, Wong N, et al. 53BP1 inhibits homologous recombination in BRCA1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243-54.
51. Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci. 2011;108:3406-11.
52. Liu J, Fleming GF, Tolaney SM, et al. A phase I trial of the PARP inhibitor olaparib (AZD2281) in combination with the antiangiogenic cediranib (AZD2171) in recurrent ovarian or triple-negative breast cancer. J Clin Oncol. 2011;29 (suppl; abstr 5028).
53. Lee J, Annunziata CM, Minasian LM, et al. Phase I study of the PARP inhibitor olaparib (O) in combination with carboplatin (C) in BRCA1/2 mutation carriers with breast (Br) or ovarian (Ov) cancer (Ca). J Clin Oncol. 2011;29(suppl; abstr 2520).
54. Moulder S, Mita M, Bradley C, et al. [P6-15-01]. A phase 1B study to assess the safety and tolerability of the PARP inhibitor iniparib (BSI-201) in combination with irinotecan for the treatment of patients with metastatic breast cancer (MBC). Cancer Res. 2010;70(24 Suppl):Abstract nr P6-15-01.
55. Drew Y, Ledermann JA, Jones A, et al. Phase II trial of the poly(ADP-ribose) polymerase (PARP) inhibitor AG-014699 in BRCA 1 and 2–mutated, advanced ovarian and/or locally advanced or metastatic breast cancer. J Clin Oncol. 2011;29(suppl; abstr 3104).
For a report on the latest research on PARP inhibitors in triple-negative breast cancer, see page 1088 in ONCOLOGY's coverage of the ASCO Breast Cancer Symposium, which appears later in this issue.