A Perimenopausal Woman With a Small, ER/HER2–Positive, Node-Negative Breast Cancer
This installment of Second Opinion examines the case of a patient with a new diagnosis of breast cancer presenting to our multidisciplinary breast cancer second opinion clinic.
ABSTRACT: Second OpinionMultidisciplinary Consultations on Challenging CasesThe University of Colorado Health Sciences Center holds weekly second opinion conferences focusing on cancer cases that represent most major cancer sites. Patients seen for second opinions are evaluated by an oncologist. Their history, pathology, and radiographs are reviewed during the multidisciplinary conference, and then specific recommendations are made. These cases are usually challenging, and these conferences provide an outstanding educational opportunity for staff, fellows, and residents in training.The second opinion conferences include actual cases from genitourinary, lung, melanoma, breast, neurosurgery, and medical oncology. On an occasional basis, ONCOLOGY will publish the more interesting cases discussions and the resultant recommendations. We would appreciated your feedback; please contact us at second.opinion@uchsc.edu.E. David Crawford, MD, and Al Barqawi, MD, Guest EditorsUniversity of Colorado Health Sciences Centerand Univeristy of Colorado Cancer CenterDenver, Colorado
This installment of Second Opinion examines the case of a patient with a new diagnosis of breast cancer presenting to our multidisciplinary breast cancer second opinion clinic.
History
A 52-year-old woman presented to our multidisciplinary clinic after a new diagnosis of invasive ductal carcinoma of the left breast. Suspicion of breast cancer was initially raised by abnormal screening mammography of the left breast 2 months prior to presentation. The woman was asymptomatic at that time, and mammography performed 15 months prior to the current presentation had been unremarkable. She was otherwise healthy.
The patient underwent lumpectomy and sentinel lymph node biopsy. Examination of the lumpectomy specimen revealed a combined histologic grade 2 invasive ductal carcinoma with a greatest tumor dimension of 7 mm and a closest resection margin of 2 mm. There was no lymphovascular invasion. The tumor was estrogen receptor (ER)-positive (60%) with low-level progesterone receptor (PR) expression (4%). HER2 immunohistochemistry (IHC) was performed and scored as 2+. Subsequent fluorescence in situ hybridization (FISH) showed a ratio of HER2 gene copy number to chromosome 17 centromere of 2.8. Three sentinel lymph nodes were negative for tumor involvement. The remainder of the disease staging work-up was negative, resulting in an overall stage of T1b, N0, M0 (stage I).
The woman presented to our clinic with this information seeking a recommendation regarding further treatment. Her physical examination and family history were unremarkable. On review of systems she noted continued menstrual bleeding, but her periods had become less frequent and more irregular over the preceding year.
Discussion
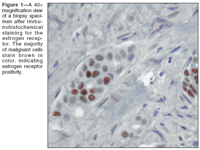
Dr. Gabriel Brooks: This case of a perimenopausal woman with a small, node-negative, ER-positive, HER2-positive tumor presents considerable challenges with respect to risk assessment and selection of appropriate therapy. Our patient has multiple favorable prognostic factors, including a tumor diameter of less than 1 cm, positive ER expression (Figure 1), and negative sentinel lymph node biopsy. Her only adverse prognostic factor is HER2 amplification. We will begin with a discussion of the pathologic characteristics of this tumor.
Tumor Pathology
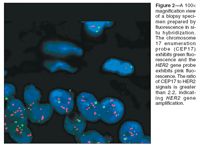
Dr. Brooks: How do we interpret the results of HER2 testing?
Dr. Meenakshi Singh (pathology): Because HER2 status is used as a basis for making decisions regarding candidacy for both conventional chemotherapy and trastuzumab (Herceptin) treatment, it is essential to have a well-validated system for clinical determination of HER2 status. On the basis of joint guidelines from the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP),[1] HER2-positive status is determined by either 3+ overexpression of the HER2 protein on IHC staining or a FISH ratio of greater than 2.2 for HER2 gene amplification (Figure 2). A HER2 IHC of 2+ or a HER2 FISH ratio between 1.8 and 2.2 is considered an equivocal result, and requires further analysis. The FISH ratio compares the number of HER2 gene copies to the number of chromosome 17 copies, as measured by the chromosome enumeration probe 17 (CEP17).
In this case, the sequence of an initial equivocal IHC result followed by FISH analysis was appropriate. This patient should be considered clinically positive for HER2 gene amplification, albeit at a low level.
Dr. Brooks: Are there any other factors that can interfere with the HER2 assay?
Dr. Singh: Appropriate tissue handling prior to testing is crucial to ensure reproducible results. The ASCO/CAP guidelines specify that time from tumor excision to fixation should be as short as possible and that tissue formalin fixation time should be at least 6 hours for excision specimens, at least 1 hour for needle-biopsy specimens, and not greater than 48 hours for either. At this institution, we ask our surgeons to record the exact times of specimen excision and placement into formalin. In the laboratory, we record the total time of specimen fixation so that we can monitor our compliance with the guidelines.
Additional factors that can confound HER2 results are polysomy of chromosome 17 and heterogeneity of HER2 amplification within a tumor. When three or more copies of chromosome 17 are present in a cell, the ratio of HER2 to CEP17 can be less than 1.8 despite a numeric increase in the number of HER2 copies. While elevated HER2 expression by IHC may be seen in this scenario,[2] the majority of cases of polysomy 17 do not overexpress HER2.[3] In cases of heterogeneity, tumors may have some areas of significant overexpression or amplification of HER2 and other areas of normal expression. Our patient did not have polysomy 17 or heterogeneity of HER2 expression.
Dr. Brooks: Our patient also has low-level expression of the progesterone receptor measured at 4%, with clearly positive estrogen receptor expression at 60%. What is the significance of low-level progesterone receptor expression?
Dr. Singh: In our laboratory, we do not report ER and PR status as negative or positive; rather, we report the percentage of cells that exhibited receptor staining by IHC, as was done in this case. The initial studies that validated PR expression as adding utility to ER expression in prediction of response to tamoxifen used quantitative radioligand binding assays to determine expression.[4,5] IHC is now the standard of care in assessing PR and ER expression, and it has been shown that response to tamoxifen improves continuously with increasing PR expression,[6] so that low-level PR expression as observed in our patient can at least be seen to predict a better response to endocrine therapy than no expression at all. As with HER2 assessment, all ER and PR testing should be carried out in an accredited lab with positive and negative controls. The clone of HER2 antibody used and the method of analysis should be clearly documented in the pathology report.
Risk Assessment
Dr. Brooks: Given the information we have, how can we best estimate this patient’s risk of disease recurrence?
Dr. Virginia Borges (medical oncology): Risk assessment begins with understanding the biologic characteristics of the tumor. This tumor expresses the estrogen receptor, has low-level progesterone expression and is HER2-positive. Preclinical studies with gene expression arrays have advanced the understanding of breast cancer as a heterogeneous disease, with multiple breast cancer subtypes classifiable by differential gene expression.[7] The expression signature of this tumor seen by IHC and FISH most nearly correlates with an overlap between the HER2 and the luminal B breast cancer subtypes, carrying a worse prognosis than the luminal A subtype.
Dr. Anthony Elias (medical oncology): While the tumor biology is of great importance in assessing the risk of recurrence, it is also important to factor in the small size of this tumor in order to avoid overestimating risk, and therefore overestimating the benefit of aggressive therapy. The available data suggest that tumors less than 1 cm in size without nodal metastases have a very favorable prognosis. The prognosis of small, HER2-positive tumors, whether ER-positive or -negative, is based on small case series largely from single institutions and is less well characterized.
Dr. Brooks: A recent study of over 50,000 patients with tumors less than 1 cm drawn from the population-based Surveillance, Epidemiology and End Results (SEER) database found a 10-year breast cancer–specific mortality of only 4%.[8] Relapse and treatment data, as well as HER2 status, were not available in this study.
Another population-based study from Finland showed an 87% 10-year distant disease–free survival among 1,208 patients with T1, N0 tumors, in spite of only 5% of these patients receiving any adjuvant therapy.[9] However, this study also suggested that HER2 amplification conferred a worse prognosis even in small tumors; 69 patients with T1, N0, HER2-positive tumors had a 9-year distant disease–free survival of 73%.
A total of 76 patients with T1a/b, N0, HER2-positive tumors treated at the Dana-Farber Cancer Institute had a 5-year disease-free survival of 90.5%.[10] These patients were more heavily treated with adjuvant therapy than the patients in the Finnish study, with rates of endocrine therapy and chemotherapy usage of 54% and 34%, respectively. None of these trials indicated the relative impact of systemic adjuvant therapy.
What other tools can we use to estimate the risk of relapse in this patient?
Dr. Borges: The Adjuvant! Online website (www.adjuvantonline.com) is a commonly used clinical resource for risk assessment in breast cancer. In this case, however, I believe Adjuvant! could have shortcomings, depending on how it is used.
Dr. Brooks: For our patient, Adjuvant! calculates a 77.2% 10-year disease-free survival, with a 2.9% breast cancer–specific mortality. Mortality and relapse data calculated by Adjuvant! use patient-specific input fields and are based on data from the SEER database and on randomized clinical trials with at least 10 years of data on disease-free and overall survival.
Dr. Borges: This estimate does not take HER2 overexpression into account (unless the provider manually enters a risk adjustment into the algorithm) and therefore likely underestimates risk for our patient. Also, it is important to keep in mind that the relapse estimate in Adjuvant! is secondarily derived from mortality data, as the SEER database does not include information on disease relapse. The relapse estimate combines both locoregional and distant relapse, making it less clinically useful in determining the benefit of adjuvant therapy for systemic recurrence.
Dr. Christina Finlayson (surgery): Another risk assessment tool available for this patient is Oncotype DX. Although it is uncommonly used in patients with HER2-positive tumors, it could be helpful in this scenario.
Dr. Brooks: Oncotype DX is a gene-expression assay validated to predict the risk of recurrence in patients with node-negative, ER-positive breast cancer who will be treated with endocrine therapy.[11] Oncotype DX uses the expression levels of 16 malignancy-associated genes and 5 genes expressed in normal tissues to calculate a recurrence score. Patients are assigned to low-risk, intermediate-risk, and high-risk groups on the basis of the recurrence score. The classification suggests that low-risk patients have no benefit from adjuvant chemotherapy and significant benefit from endocrine therapy, whereas high-risk patients demonstrate the reverse-clear benefit from chemotherapy and minimal benefit from endocrine therapy.[12,13]
Dr. Elias: Because elevated expression levels of HER2 and another HER2-associated gene, GRB7, are both used in the recurrence score calculation, Oncotype DX is usually deemed to be a redundant test in patients with documented HER2 overexpression or amplification. In fact, a recent analysis showed that tumors with high levels of HER2 mRNA had a median recurrence score of 32 even though ER-positive.[14] Given our patient’s observed HER2 amplification, I think it is unlikely that a recurrence score would classify her as low-risk.
Dr. Borges: I agree that it is unlikely that she will be found to be low risk, but I would consider getting the recurrence score. This may serve as a double-check of the somewhat equivocal results of the pathology, which showed indeterminate status of HER2 overexpression by IHC and a borderline positive result for HER2 gene amplification by FISH.
Dr. Brooks: The cost of the Oncotype DX assay is approximately $3,500. Assuming that this information would influence whether or not chemotherapy was offered and hypothesizing that there is a 5% to 10% probability of assignment to a low-risk group, the cost of this test would be $35,000 to $70,000 for each clinically informative result. Since the assay was validated in a broad population of ER-positive breast cancers, among which HER2-positive tumors comprise only 4%, it is still unknown if the assay has the same predictive characteristics in this particular population. More studies are needed on the utility of the Oncotype DX assay in women with ER/HER2–positive tumors.
At this point, how can we best summarize this patient’s risk of relapse?
Dr. Elias: This woman’s prognosis is not well defined by the existing literature. Adjuvant! Online is largely uninformative. The benefits of performing the Oncotype DX assay are unclear. The best evidence for estimating her prognosis is based largely on the existing case series that documented survival in women with small, HER2-positive tumors, including those series mentioned previously and other smaller series.[9,10,15,16] In review of the available literature, her prognosis for disease-free survival ranges from as low as 70% to as high as 96% at 10 years, after accounting for the heterogeneous therapy these patients have been given.
Locoregional Therapy
Dr. Brooks: In evaluating this patient’s risk of recurrence we have concentrated on the biologic characteristics of her tumor. Do these characteristics affect the selection of appropriate surgical or radiologic therapy?
Dr. Finlayson: This woman had a lumpectomy with negative resection margins around a small tumor. Her sentinel lymph node biopsy was negative. Although the adverse biologic characteristics of her tumor are likely to increase the risk of local recurrence, I do not recommend any further surgical intervention at this time. As part of counseling prior to her lumpectomy, I would have advised her of the slightly increased risk of local recurrence associated with her tumor and offered her the option of mastectomy to reduce this risk of local recurrence. Having said this, breast-conservation therapy is entirely appropriate in this case.
Dr. Rachel Rabinovitch (radiation oncology): Standard-of-care local therapy for this woman consists of whole-breast irradiation (WBI) as part of breast-conservation therapy. The patient is both ER-positive and HER2-positive, a more favorable subtype than either ER/PR–negative, HER2-positive tumors or triple-negative tumors. Nguyen et al recently published a very elegant analysis of the impact of varying ER/PR and HER2 combinations on local and distant therapy.[17] Their assessment demonstrated no increased risk of local failure for an ER/PR–positive/HER2-positive patient compared to a patient with ER/PR–positive/HER2-negative disease, while both triple-negative tumors and ER/PR–negative, HER2-positive tumors were found to have elevated local relapse rates compared to ER/PR–positive, HER2-negative tumors. This patient’s HER2 status should not be used to argue against the appropriateness of breast-conserving therapy.
Although WBI is standard therapy and will reduce the woman’s risk of ipsilateral breast tumor recurrence, her overall likelihood of local recurrence without radiotherapy is small. This is illustrated by a Canadian trial conducted in a population of women aged 50 years and older with tumors less than 5 cm in diameter treated with lumpectomy and tamoxifen and randomized to WBI or observation.[18] Updated results of this study from 2006 found a 4.4% rate of local relapse at 8 years among patients with T1 ER-positive tumors treated with radiation and tamoxifen, compared to a 9.9% rate among patients receiving tamoxifen alone. For women with ER-positive tumors less than 1 cm, the difference between the two groups was less than 3% (4% vs 6.7%). This demonstrates both the low risk of recurrence at 8 years and the consistent reduction of that risk with WBI-on the order of 50%. Our patient is very young and likely to live another 30 years or so in the absence of serious comorbidities; her lifetime risk of breast recurrence would therefore be three to four times that rate without adjuvant radiotherapy, and so, such an approach would not be advisable.
A fair amount of data demonstrate that alternative accelerated fractionation schemes are available to treat our patient. With supportive evidence from the randomized Canadian trial conducted by Whelan et al and the recently published Standardisation of Breast Radiotherapy (START) B trial from the UK,[19,20] I treat nearly all my node-negative patients receiving WBI with accelerated fractionation, typically to a total dose of 42.5 Gy in 16 fractions. This reduces the time for WBI from 5 to 3 weeks, and yields equivalent local control and cosmesis compared to the traditional regimen. I also administer a lumpectomy-bed boost to nearly all patients, supported by the European Organisation for Research and Treatment of Cancer (EORTC) trial results.[21] Admittedly, none of the published accelerated WBI trials utilized a boost, however.
Another consideration for this patient would be partial-breast irradiation (PBI), although I don’t consider this a standard treatment option at this time. PBI treats only a limited volume of tissue around the lumpectomy bed in twice-daily fractions over 5 days of treatment. Doses per fraction are larger than most WBI schedules, with lower total doses. PBI can be administered via interstitial brachytherapy, intracavitary brachytherapy, or three-dimensional conformal external-beam techniques.[22]
If our patient were interested in PBI, she should understand that there is minimal long-term data for brachytherapy series, though the largest reports demonstrate very good results. There is essentially no published follow-up of patients treated with external-beam PBI for any significant length of time. National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 is a randomized trial currently enrolling patients to compare these PBI treatments to WBI. Our patient would not be eligible for the trial, as participation by low-risk patients is currently closed. If chosen, PBI should be administered before any planned chemotherapy, in contrast to WBI, which is delivered after the completion of chemotherapy. Trastuzumab or endocrine therapy can be given concurrently with radiotherapy in any form.
Adjuvant Endocrine Therapy
Dr. Borges: Endocrine therapy is an essential component of adjuvant therapy for ER-positive breast cancer. The decision of which endocrine therapy to choose for our patient is complicated by her perimenopausal status. Confirming a woman’s menopausal state can be challenging, and the National Comprehensive Cancer Network (NCCN) breast cancer guidelines give a helpful algorithm for making this determination.[23]
Dr. Elias: I agree that endocrine therapy is essential for this patient. She is 52 years old and perimenopausal. Although aromatase inhibitors have exhibited consistent superiority to tamoxifen in reducing recurrence of ER-positive breast cancer,[24-27] these agents are contraindicated in women with functional ovaries because they can cause central hyperstimulation of ovarian function. Therefore, the treatment options for this perimenopausal woman include tamoxifen for 5 years or tamoxifen followed by crossover to an aromatase inhibitor after menopause or ovarian ablation.
Dr. Brooks: Our patient’s tumor has poor expression of the progesterone receptor as well as HER2 overexpression, both of which have been posited as predictors of poor response to tamoxifen.[28] Should these adverse biologic characteristics of the tumor affect our enthusiasm for endocrine therapy?
Dr. Elias: I do not think that the available data suggest that her PR-poor, HER2-positive status should affect our endocrine therapy selection. An update to the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview data[29] presented at the 2007 ASCO meeting partly dispelled concerns about tamoxifen resistance in PR-poor tumors. Although the overview data confirm a worse overall prognosis for ER-positive/PR-poor disease compared to ER/PR–positive disease, tamoxifen treatment produces the same relative risk reduction of approximately 40% in both groups.
In the Genomic Health Inc database, ER mRNA levels correlated with response to tamoxifen therapy as a predictive but not a prognostic factor, while conversely, a low PR mRNA level correlated with the biologic aggressiveness of the tumor as an adverse prognostic factor but was not predictive of lack of benefit to tamoxifen therapy.[12] More information is needed regarding the relative efficacy of tamoxifen in patients with HER2 overexpression; however, there are no compelling data that this patient population is excluded from the expected benefits of endocrine therapy.
Dr. Borges: I would recommend tamoxifen therapy with crossover to an aromatase inhibitor after 2 to 3 years, assuming our patient is confirmed to be postmenopausal at that time. Enrollment in the Suppression of Ovarian Function Trial (SOFT) would also be a reasonable option. SOFT is a three-arm trial of tamoxifen, ovarian ablation with tamoxifen, or ovarian ablation with exemestane (Aromasin) in premenopausal patients, with each treatment course lasting 5 years.
During endocrine therapy, our patient should be followed closely for any loss of bone mineral density. The utility of bisphosphonates as preventive treatment for metastatic bony disease is an area of great interest. An initial study published 10 years ago showed a reduction in bony metastases with the use of bisphosphonates as adjuvant therapy in patients with breast cancer,[30] but this has not been consistently replicated.[31] New data have just emerged in abstract form from the Austrian Breast and Colorectal Cancer Study Group (ABCSG) 12 trial, in which 1,801 premenopausal women with stage I/II endocrine-responsive breast cancer were randomized to receive zoledronic acid (Zometa, Reclast) every 6 months for 3 years vs no scheduled bisphosphonate therapy. Subjects receiving zoledronic acid had a 36% reduction in disease-free survival events after a median of 5 years of follow-up. [32]
As our understanding of the role of bisphosphonates in the adjuvant therapy of breast cancer matures, I would encourage our patient to enroll in a clinical trial that would help better define this role. The S0307 is one such open trial, comparing the early-generation oral bisphosphonate clodronate (available as Bonefos and Loron in the United Kingdom and Canada) against the more potent oral agent ibandronate (Boniva) and the intravenous agent zoledronate (Zometa, Zabel), each for 3 years.
Adjuvant Chemotherapy
Dr. Brooks: The selection of an appropriate chemotherapy regimen for this patient, or of no chemotherapy at all, is the most difficult decision posed by this case. Here the treatment guidelines for adjuvant chemotherapy in breast cancer offer divergent recommendations. Relevant guidelines from the NCCN state that adjuvant endocrine therapy should be given with the “consideration” of adjuvant chemotherapy.[23] Biologic therapy with trastuzumab is not recommended. Under these guidelines, the use of trastuzumab is reserved for the patient population that was included in reported clinical trials of adjuvant trastuzumab-ie, patients with positive nodes or tumors greater than 1 cm. In contrast, the St. Gallen’s consensus statement places this patient at intermediate risk on the basis of HER2 overexpression, and recommends treatment with both endocrine therapy and chemotherapy including trastuzumab. [33] Is this patient likely to benefit from chemotherapy?
Dr. Elias: We estimated her 10-year disease-free survival as 70% to 96% previously (with systemic therapy undefined), anticipating a 40% improvement in relative risk of relapse after endocrine therapy. The benefit of conventional chemotherapy is likely to result in an additional 25% to 56% relative reduction of the risk of relapse, with the inherent potential for significant therapy-related morbidity. Given the targeted nature of trastuzumab and its remarkable success in addition to chemotherapy in four large adjuvant trials, it is tempting to offer this patient a HER2-specific regimen such as TCH (docetaxel [Taxotere], carboplatin, trastuzumab). Thinking outside the box, perhaps a purely targeted approach with complete antiestrogen therapy and blockade of the HER2 axis with or without chemotherapy might make the most sense, although no clinical data are available for this scenario.
Dr. Brooks: NSABP B-31, North Central Cancer Treatment Group (NCCTG) N-9831, the Herceptin Adjuvant (HERA) study, and Breast Cancer International Research Group (BCIRG) 006 are all large, randomized controlled trials of chemotherapy with or without trastuzumab for patients with HER2 overexpression.[34-36] These trials predominantly included patients with node-positive disease, although HERA and BCIRG 006 both included significant numbers of “high-risk” node-negative patients. All node-negative patients in the HERA investigation had primary tumors greater than 1 cm; however, our patient would have met criteria for inclusion in BCIRG 006. Because results from BCIRG 006 have only been reported in interim abstract form, no details are available on the few patients in that study who had T1b, N0 disease. In all four trials, the addition of trastuzumab to chemotherapy translated to a significant improvement in disease-free survival, with consistent findings of a 30% to 50% relative reduction in risk of recurrence over chemotherapy alone.
Dr. Borges: I would discuss chemotherapy with this patient because she is young and otherwise healthy, although I would emphasize our limited understanding of her breast cancer subset and of what degree of benefit therapy would offer. Whether or not to incorporate trastuzumab is also an unknown. If her tumor had other negative prognostic factors, such as poor grade, then with the mass at this size I would feel more strongly about moving forward with chemotherapy and trastuzumab according to one of the approved adjuvant regimens.
At present, the only therapy we know she is expected to benefit from is the antiendocrine agent. Given the data from the metastatic setting and the completed adjuvant trials, the use of chemotherapy alone would be counter to our understanding of HER2-based disease treatment. Because of both the increased risks of combining conventional chemotherapy with trastuzumab and the unknown efficacy of such a regimen in this breast cancer subpopulation, such a combination regimen is not recommended for our patient under current guidelines. Studies with combined HER2-targeted therapy and endocrine therapy would be ideal in this patient population.
Regarding the choice of a conventional chemotherapy regimen, previous data have suggested that HER2 overexpression is a marker for tumor sensitivity to anthracycline-containing regimens.[37] The biologic mechanism of this sensitivity is not clear, although topoisomerase II is a known target of anthracyclines and is coamplified with HER2 in about 35% of HER2-positive breast cancers. It is possible that anthracycline sensitivity only applies to the subset of HER2-positive patients who also overexpress topoisomerase II.
More data on this topic will be available with the publication of the BCIRG 006 trial of adjuvant trastuzumab, which will include subgroup analysis by topoisomerase II amplification status. In the meantime enrollment of patients like this one in clinical trials of trastuzumab-based therapy is of critical importance. The only such open trial for which our patient would be eligible is a phase II open-label trial of paclitaxel and trastuzumab for patients with stage I node-negative breast cancer.
Dr. Elias: Weighing the risks of chemotherapy against an expected benefit of 2% to 4% absolute reduction in risk of breast cancer recurrence at 10 years, I am hesitant to recommend any treatment in addition to endocrine therapy. While gains in breast cancer survival are rightly attributed to the accumulation of small incremental benefits, these must be weighed against morbidities when applied to individual patients. Further trials in this patient subgroup are necessary to be able to offer more evidence-based recommendations in the future. Trial concepts include adjuvant therapies with a taxane and trastuzumab followed by antiestrogen therapy, or lapatinib (Tykerb) plus antiestrogen therapy. If this patient feels strongly about pursuing aggressive treatment, I would then treat her according to the BCIRG 006 trial using the docetaxel, carboplatin, and trastuzumab regimen.
Final Treatment Recommendations
Completion of breast-conservation therapy with accelerated whole-breast irradiation and 5 years of endocrine therapy (tamoxifen with crossover to an aromatase inhibitor after the onset of menopause) were unanimously recommended. Recommendations for additional adjuvant systemic therapy with chemotherapy or trastuzumab were less clear-cut, reflecting the lack of clear evidence in favor of or against additional systemic intervention for patients with small, ER/HER–positive tumors. Both medical oncologists entertained the options of either endocrine therapy alone or endocrine therapy and treatment with a combined regimen of conventional chemotherapy and trastuzumab, either by the BCIRG 006 protocol or on an open investigational protocol.
References:
References
1. Wolff A, Hammond M, Schwartz J, et al: American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118-145, 2007.
2. Lear-Kaul K, Yoon H, Kleinschmidt-DeMasters B, et al: HER-2/neu status in breast cancer metastases to the central nervous system. Arch Pathol Lab Med 127:1451-1457, 2003.
3. Downs-Kelly E, Yoder B, Stoler M, et al: The influence of polysomy 17 on her2 gene and protein expression in adenocarcinoma of the breast: A fluorescent in situ hybridization, immunohistochemical, and isotopic MRNA in situ hybridization study. Am J Surg Pathol 29:1221-1227, 2005.
4. Ravdin P, Green S, Dorr T, et al: Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: Results of a prospective Southwest Oncology Group study. J Clin Oncol 10:1284-1291, 1992.
5. Bardou V, Arpino G, Elledge R, et al: Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21:1973-1979, 2003.
6. Elledge R, Green S, Pugh R, et al: Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and PS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: A Southwest Oncology Group study. Int J Cancer 89:111-117, 2000.
7. Brenton J, Carey L, Ahmed A, et al: Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J Clin Oncol 23:7350-7360, 2005.
8. Hanrahan E, Gonzalez-Angulo A, Giordano S, et al: Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol 25:4952-4960, 2007.
9. Joensuu H, Isola J, Lundin M, et al: Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: A nationwide population-based study. Clin Cancer Res 9:923-930, 2003.
10. Black D, Younger J, Martei Y, et al: Recurrence risk in T1a-b, node negative, HER2 positive breast cancer (abstract 2037). Breast Cancer Res Treat 100(suppl 1):S92, 2006.
11. Paik S, Shak S, Tang G, et al: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817-2826, 2004.
12. Paik S, Shak S, Tang G, et al: Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer (abstract 510). J Clin Oncol 23(16S):6s, 2005.
13. Paik S, Tang G, Shak S, et al: Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726-3734, 2006.
14. Watson D, Palmer G, Baker J, et al: The impact on the recurrence score due to patient variation in the quantitative expression of individual genes or gene groups (abstract 6039). Breast Cancer Res Treat 100(suppl 1):S269, 2006.
15. Norris B, Chia S, Cheang M, et al: Poor 20 yr breast cancer specific survival and relapse free survival for HER2 positive T1N0 tumors (abstract 2031). Breast Cancer Res Treat 100(suppl 1):S90, 2006.
16. Amar S, Ann M, Geiger X, et al: Prognosis and clinical outcome of patients with node negative 1cm breast cancer (abstract 6024). Breast Cancer Res Treat 106(suppl 1):S254, 2007.
17. Nguyen P, Taghian A, Katz M, et al: Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 26:2373-2378, 2008.
18. Fyles A, McCready D, Manchul L, et al: Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 351:963-970, 2004.
19. Whelan T, MacKenzie R, Julian J, et al: Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 94:1143-1150, 2002.
20. START Trialists’ Group: The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 371:1098-1107, 2008.
21. Bartelink H, Horiot J, Poortmans P, et al: Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med 345:1378-1387, 2001.
22. Orecchia R, Fossati P: Partial breast irradiation: Ready for routine? Breast 16(suppl 2):S89-S97, 2007.
23. Carlson R, Allred D, Anderson B, et al: NCCN clinical practice guidelines in oncology: Breast cancer (version 2.2008). Available at www.nccn.org. Accessed August 28, 2008.
24. Howell A, Cuzick J, Baum M, et al: Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60-62, 2005.
25. Coombes R, Hall E, Gibson L, et al: A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081-1092, 2004.
26. Kaufmann M, Jonat W, Hilfrich J, et al: Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: The ARNO 95 study. J Clin Oncol 25:2664-2670, 2007.
27. Breast International Group (BIG) 1-98 Collaborative Group: A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747-2757, 2005.
28. Arpino G, Weiss H, Lee A, et al: Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97:1254-1261, 2005.
29. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687-1717, 2005.
30. Diel I, Solomayer E, Costa S, et al: Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med 339:357-363, 1998.
31. Hillner B, Ingle J, Chlebowski R, et al: American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21:4042-4057, 2003.
32. Gnant M, Mlineritsch B, Schippinger W, et al: Adjuvant ovarian suppression combined with tamoxifen or anastrozole, alone or in combination with zoledronic acid, in premenopausal women with hormone-responsive, stage I and II breast cancer: First efficacy results from ABCSG-12 (abstract LBA4). J Clin Oncol 26(15S):6s, 2008.
33. Goldhirsch A, Wood W, Gelber R, et al: Progress and promise: Highlights of the International Expert Consensus on the Primary Therapy of Early Breast Cancer 2007. Ann Oncol 18:1133-1144, 2007.
34. Romond E, Perez E, Bryant J, et al: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673-1684, 2005.
35. Smith I, Procter M, Gelber R, et al: 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet 369:29-36, 2007.
36. Slamon D, Eiermann W, Robert N, et al: BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACâT) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ACâTH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2Neu positive early breast cancer patients (abstract 52). Proceedings of the 29th Annual San Antonio Breast Cancer Symposium, San Antonio, Texas, December 14-16, 2006.
37. Pritchard K, Messersmith H, Elavathil L, et al: HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol 26:736-744, 2008.