Perverse financial incentives tip usage in favor of IV drugs
More than a decade has passed since the FDA approved the first pill to fight cancer. Designed to battle metastatic colorectal cancer, capecitabine (Xeloda) marked a significant change in chemotherapy, untethering some cancer patients from office-based intravenous drug infusions. Other such drugs have since been commercially released, including temozolomide (Temodar) and imatinib (Gleevec), but the reimbursement system in this country has failed to keep up.
ABSTRACT: The coming wave of oral antineoplastics has set the stage for a shift in payer coverage. But restructuring insurance plans to cover IV and oral drugs more equitably may not be a total solution.

More than a decade has passed since the FDA approved the first pill to fight cancer. Designed to battle metastatic colorectal cancer, capecitabine (Xeloda) marked a significant change in chemotherapy, untethering some cancer patients from office-based intravenous drug infusions. Other such drugs have since been commercially released, including temozolomide (Temodar) and imatinib (Gleevec), but the reimbursement system in this country has failed to keep up.
Intravenous infusions continue to dominate patient choices for chemotherapy, even when oral agents promise to do at least as well. One reason is an economic disincentive against the use of these oral oncolytics.
Insurers are willing to pay tens of thousands of dollars a year for individual IV therapies, placing only a small proportion, if any, of the financial burden on patients. But oral chemotherapeutics are often accompanied by copayments that reach deep into patients’ pockets.
“IV drugs typically are covered under major medical which may not have as many payment restrictions as they do on oral drugs, which are generally a pharmacy benefit,” said Joseph S. Bailes, MD, chair of the ASCO government relations committee.

A substantial hurdle
Such economic disincentives against oral anticancer agents exist, even though pills offer advantages over IV infusions. And payers know it. In a survey conducted by the market research firm Reimbursement Intelligence, managed care medical and pharmacy directors from top U.S. health plans acknowledged that pills are more convenient for patients than IV drugs. They also stated that patients generally do better when they spend less time in the hospital or at an infusion center. Making it easier for patients to choose oral medications could even help payers by helping them track pharmacy benefit data for drug utilization reviews and cost management analyses.
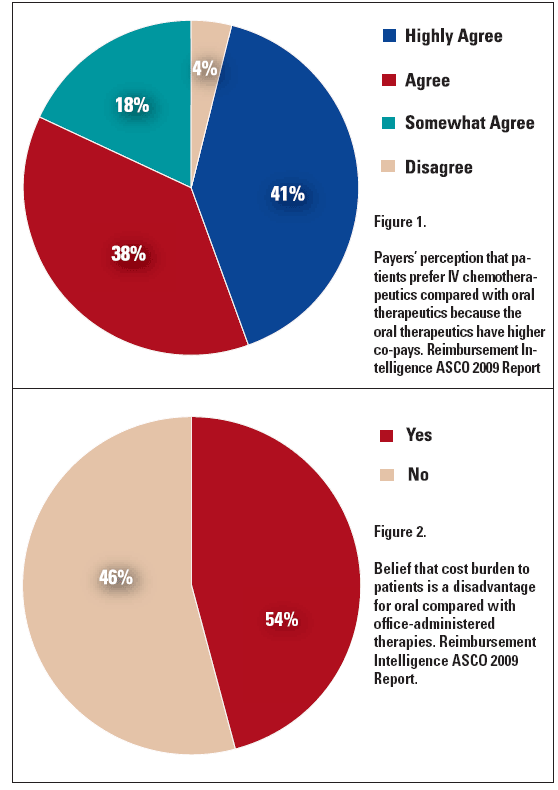
The majority of insurers are also aware that the current reimbursement system serves as a substantial hurdle to the use of oral agents. In a survey of 60 decision makers representing the top health plans in the U.S., Reimbursement Intelligence found that 96% agreed (highly to somewhat) that patients prefer oral agents over IV cancer drugs. The survey also found that 54% of respondents believe that the cost burden to patients is a disadvantage for oral therapies versus office-administered therapies. One respondent opined that “some patients may prefer an IV drug because it is part of the medical benefit and orals are included in pharmacy benefit, which usually comes with a higher copay” (see Figure 1 and Figure 2 below).
Looking at the other side of the coin, Reimbursement Intelligence found that between 60% and 80% of oncology dollars are spent through the medical benefits side and the remainder through pharmacy. This, in itself, is a key reimbursement hurdle for oral agents, according to the report.
The doughnut hole
The problem stems at least partly from the way insurance coverage is set up. Because a year’s supply of oral medication may cost between $20,000 and $40,000, hefty copays under the pharmacy benefit put these alternatives beyond the economic grasp of many patients.
This is especially so for elderly patients covered under Medicare Part D, which has a 25% copay for prescription drugs and an annual doughnut hole that appears after Medicare patients chew through their first $2,700 or so of their prescriptions. This hole sticks patients with thousands of dollars in prescription bills before coverage kicks in again. “It is unlikely that payers will say they will lower the copay or coinsurance on oral agents,” said Rhonda Greenapple, president of Reimbursement Intelligence.
Restructuring insurance plans to cover IV and oral drugs more equitably may not be a total solution, because the problem is not nearly as simple as it may seem. Oral and IV drugs are generally not interchangeable, according to Dr. Bailes. “It’s really apples and oranges,” he said. “The use of a drug is determined by the clinical trial and the tumor type. You don’t get head-to-head comparisons of oral and IV drugs in clinical trials.”
An example is lapatinib (Tykerb), he said. In combination with capecitabine and following prior therapy including an anthracycline, a taxane, and trastuzumab (Herceptin), lapatinib has proved to be effective against HER2-positive metastatic breast cancer. But this combination was not specifically tested head-to-head against existing IV medications.

A question of compliance
There are other shortcomings to a regimen based on pills. Compliance is one of them. Some patients may take a lower than prescribed dose. Whether they are trying to stretch their prescriptions to save money or just have trouble keeping a complex regimen of medications straight is open to debate. But the reason doesn’t matter as much as the effect: suboptimal dosing and potentially diminished clinical effect.
Advocates of infused oncolytics also argue persuasively that patients taking pills may not notice adverse effects that would be apparent to a physician whose supervision would also ensure that the medication, if administered intravenously, would be given in the appropriate dose.
These caveats have not stopped legislative efforts, however, to try to minimize the economic disincentives currently built into the system.

Oregon, Hawaii, and Indiana have passed laws requiring insurance companies to provide the same coverage for oral and IV antineoplastics, according to the National Patient Advocate Foundation. Several other states, including Colorado, Minnesota, Montana, Oklahoma, and Washington, are considering similar legislation.
At the federal level, Rep. Brian Higgins (D-N.Y.) introduced HR 2366, which would create parity among oral and IV anticancer medications. The legislation has one cosponsor in the House of Representatives, according to the foundation. A Senate version has not yet been introduced.
Whether federal legislation will be enacted in the near future may depend on the success of President Barack Obama and his administration’s efforts to reform healthcare, said Nancy Davenport-Ennis, founder of the National Patient Advocate Foundation and its current president and CEO. These efforts seem to be shifting lately toward health insurance reform, a move that could help the foundation’s agenda regarding equitable reimbursement of IV and oral drugs, she said.
“This matter is squarely in the court of insurance issues, and we would be supportive of trying to move it into the larger reform bill,” Ms. Davenport-Ennis said.
Where the economic playing field remains uneven, other means for putting oral alternatives into the hands of patients have popped up. Some pharmaceutical companies provide their oral anticancer drugs free to insurance-challenged or otherwise financially needy patients. Philanthropic organiziations provide relief as well. And, depending on the plan, some insurers set an annual ceiling for patients’ total out-of-pocket expenditures for oral oncolytics.
Specialty classification
Recognizing the inequities in coverage due to the different classifications, payers are beginning to lean toward moving the coverage of all anticancer agents, regardless of the route of administration, into a specialty pharmacy classification (see “Oral chemotherapy poses more challenges for cancer community,” page 24, June 2009).
“Payers believe that specialty pharmacies will grow over the next two years and part of that will be driven by the fact that there will be a lot of oral agents coming into the market,” according to Ms. Greenapple.
Oral agents account for only about 10% of the total chemotherapies in use today. However, oral oncolytics now in the research and development pipeline could double the number of such agents in the next few years.