The Place of Transplantation in Mantle Cell Lymphoma
The role of transplant in MCL is in clinical evolution. Up-front high-dose therapy and autologous stem cell transplant remains an attractive option for those with chemosensitive disease regardless of the induction regimen chosen, whereas this approach in the relapsed or refractory setting has not yielded long-term disease-free intervals.
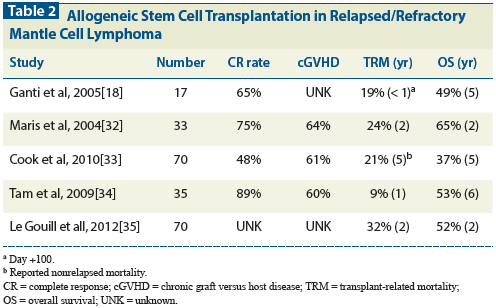
Table 1: Induction Plus Autologous Stem Cell Transplantation in the Rituximab Era
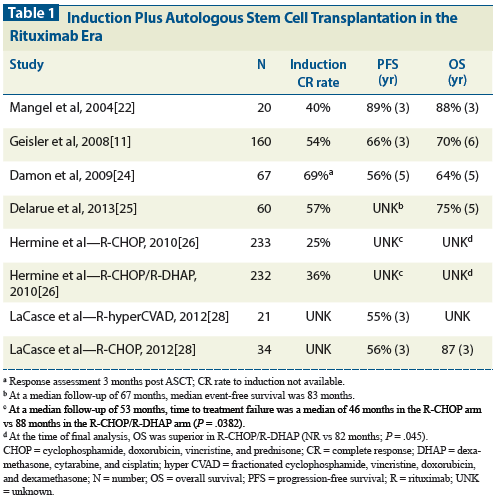
Table 2: Allogeneic Stem Cell Transplantation in Relapsed/Refractory Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) is an uncommon non-Hodgkin lymphoma with a heterogeneous natural history. Significant strides have been made in the management of MCL. Clinical follow-up exceeds a decade with long-term remission durations in some patients. Modern induction strategies employ rituximab; a cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-like backbone; and cytarabine (in alternating or sequential regimens). However, bendamustine/rituximab therapy is challenging these induction strategies. The role of transplant is in clinical evolution. Up-front high-dose therapy and autologous stem cell transplant remains an attractive option for those with chemosensitive disease regardless of the induction regimen chosen, whereas this approach in the relapsed or refractory setting has not yielded long-term disease-free intervals. Reduced-intensity allogeneic stem cell transplant remains a viable option in those with relapsed or refractory MCL.
Introduction
Mantle cell lymphoma (MCL) represents 6% of all cases of B-cell non-Hodgkin lymphoma (NHL) and affects approximately 4,000 persons each year in the United States.[1,2] Similar to chronic lymphocytic leukemia, MCL expresses the mature B-cell markers CD19 and CD20 and aberrantly expresses CD5, a characteristic T-cell marker.[3] These pathologic characteristics shrouded the diagnosis of MCL until the early 1990s, when the translocation of t(11;14)(q13;q32) was identified and later found to elicit deregulation of cyclin D1.[4,5] Subsequently, the immunohistochemical stain for cyclin D1 became clinically available, which allowed more reliable diagnosis of MCL.[6,7]
The clinical behavior of MCL is heterogeneous, unlike its clinicopathologic characteristics: men are primarily affected (75% to 80% of patients), and the majority present with advanced-stage disease. The MCL International Prognostic Index (MIPI) categorizes patients with newly diagnosed disease into three risk groups: low (44% of patients, median survival not reached), intermediate (35%; median survival, 51 months), and high (21%; median survival, 29 months) based on age, performance status, lactate dehydrogenase levels, and white blood cell count.[8]
MCL is generally an incurable malignancy with a high rate of response to therapy but an historically discouraging duration of remission. Modern induction regimens for MCL vary but often employ rituximab plus multiagent chemotherapy. Rituximab in addition to cyclophosphamide, vincristine, doxorubicin, and prednisone (R-CHOP)-the current standard of care for diffuse large B-cell lymphoma-has high overall response rates but short progression-free survival (PFS).[9] Intensified multiagent regimens are now common induction therapies for transplant-eligible patients with MCL, including R-hyperCVAD (rituximab, fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) alternating with rituximab, high-dose methotrexate, and cytarabine)[10]; intensified (maxi) R-CHOP with alternating high-dose cytarabine[11]; and the cytarabine-containing regimen DHAP (dexamethasone, cytarabine, and cisplatin), replacing an anthracycline-based approach.[12] These regimens have yielded promising responses that afford patients an option to proceed to consolidative high-dose therapy and autologous stem cell transplant (HDT-ASCT). In those considered not to be optimal candidates for HDT-ASCT, induction with R-CHOP followed by maintenance rituximab therapy provided an overall survival (OS) advantage in a phase III randomized trial when compared with R-FC (rituximab plus fludarabine and cyclophosphamide) and maintenance interferon alfa.[13]
Large retrospective studies have demonstrated that OS for patients with MCL is improving.[14] However, the place of autologous and allogeneic stem cell transplant (alloSCT) remains a topic of considerable debate.
Pre-Rituximab Experience
More than 2 decades ago, physicians at the University of Nebraska Medical Center (UNMC) were among the first to report outcomes in patients with relapsed and refractory MCL who had chemosensitive disease consolidated with HDT-ASCT.[15] A disappointing 2-year event-free survival (EFS) of 36% was seen in a cohort of 34 patients. Those who had received more than two regimens had a 2-year EFS of 0%, and those who had received two or fewer had a 2-year EFS of 45%.
A group at MD Anderson Cancer Center reported that 20 patients with previously treated MCL had obtained a complete response (CR) with salvage hyperCVAD followed by consolidative HDT-ASCT. The 3-year EFS and OS in the HDT-ASCT cohort of patients were 17% and 25%, respectively.[16] However, in the same study, untreated patients who underwent the same approach had significantly improved 3-year EFS and OS of 72% and 92%, respectively.
These results and others in the relapsed and refractory setting are considered by most to be disappointing, with no clear stabilization of the EFS/PFS curves.[15-18] The success with an up-front consolidation approach was replicated in a larger series from European and North American registries (N = 195). Patients derived a significant PFS (hazard ratio = 2.95; P = .007) and OS (hazard ratio = 2.99; P =.001) benefit if they received consolidative HDT-ASCT following a CR to induction therapy when compared with those treated later in the disease course.[19]
The European Mantle Cell Lymphoma Network performed the only randomized attempt to assess the application of HDT-ASCT in first chemosensitive response using a CHOP backbone for induction.[20,21] Patients with chemosensitive disease (N = 122) were subsequently randomized to interferon alfa (n = 60) or HDT-ASCT (n = 62) after four cycles of therapy. In the intent-to-treat analysis, the median OS was higher in the HDT-ASCT group (7.5 years vs 5.3 years; P = .031). Furthermore, in an unplanned subset analysis of the HDT-ASCT group, patients with a CR did significantly better than those with chemosensitive but persistent disease. Thus, it became apparent in the pre-rituximab era that induction therapy followed by HDT-ASCT was an attractive approach that provided remission durability for those with a CR to initial chemotherapy. This concept has not faded in the rituximab era for transplant-eligible patients.
Rituximab Era
The addition of rituximab to established induction regimens for newly diagnosed MCL has likely increased the transplant-eligible pool. Because MCL is uncommon, despite well-recognized diagnostic criteria, few randomized trials have compared induction strategies, let alone established the role of consolidative HDT-ASCT (Table 1). An early–rituximab era pair-matched retrospective analysis from Canada demonstrated superior outcomes in patients treated with a single dose of rituximab as part of stem cell purging and two post-transplant maintenance doses following either an anthracycline or cyclophosphamide/fludarabine induction regimen plus HDT-ASCT (n = 20) vs standard non-rituximab combination chemotherapy without HDT-ASCT (n = 40). The rituximab plus HDT-ASCT arm demonstrated a 3-year PFS of 89% vs 21% in the control arm.[22]
The Nordic Lymphoma Group and the Cancer and Leukemia Group B (CALGB) reported long-term study outcomes. In the MCL-2 study led by the Nordic Lymphoma Group, 160 patients were treated with rituximab plus the intensified (maxi)-CHOP/cytarabine alternating regimen followed by HDT-ASCT.[11,23] The dose of cytarabine used in the alternating regimen was 3 g/m2, not unlike what is used with part B of hyperCVAD (cytarabine, 2 g/m2). At a median follow-up of 6.5 years among survivors, the 10-year EFS and OS were 43% and 58%, respectively. The CALGB prospectively studied in 78 patients a rituximab and methotrexate plus intensified CHOP regimen with cytarabine-containing mobilization followed by HDT-ASCT.[24] This study included two doses of post-ASCT rituximab maintenance therapy. With a median follow-up of more than 4 years, the 5-year PFS and OS were 56% and 64%, respectively. Notably, the CALGB regimen did not include a significant cytarabine component. Sequential regimens have also been studied.
Recently, the Groupe d’Ãtude des Lymphomes de l’Adulte reported the results of a phase II study of R-CHOP followed by rituximab plus DHAP and HDT-ASCT in chemosensitive patients.[25] Sixty patients were treated with a sequential regimen; this strategy achieved a CR rate of 57% at the end of planned therapy and a 5-year OS of 75%. Notably, a CR was seen in only 12% of patients after receiving CHOP for three cycles plus one dose of rituximab.
A more recent study in 497 patients < 65 years of age with MCL randomized participants to R-CHOP plus HDT-ASCT (arm A) or sequential R-CHOP (three cycles) followed by R-DHAP (three cycles) plus high-dose cytarabine-containing conditioning and ASCT (arm B).[26,27] A higher CR rate was seen in arm B-54%-than in arm A-36% (P = .0003). Despite similar outcomes immediately after HDT-ASCT, arm B had a superior remission duration (49 months in arm A vs 84 months in arm B; P = .0001).
Within the limitations of cross-study comparisons, the CALGB and European approaches are comparable. These studies allow some insight into the importance of rituximab in MCL induction regimens, question the role of maintenance rituximab therapy, and validate the use of high-dose (2 g/m2 or 3 g/m2) cytarabine-containing regimens. Alternating R-CHOP/cytarabine-containing therapy has yet to be compared with sequential R-CHOP followed by cytarabine-containing therapy.
A retrospective analysis by institutions within the National Comprehensive Cancer Network (NCCN) reviewed the outcomes of 167 younger (age < 65) patients with newly diagnosed MCL who had undergone induction with R-CHOP or R-HyperCVAD with or without HDT-ASCT.[28] The cohort that received R-CHOP without HDT-ASCT (n = 29) did significantly worse than the other three cohorts. Interestingly, those who received R-hyperCVAD alone (n = 83) did as well as those who received R-CHOP plus HDT-ASCT (n = 34) and those who received R-hyperCVAD plus HDT-ASCT (n = 21). These results support those of the MD Anderson group’s earlier study of the use of R-hyperCVAD alone for six to eight cycles.[10] The MD Anderson results were nearly replicated in a cooperative group experience (Southwest Oncology Group [SWOG] 0213), but high-grade toxicity led to discontinuation of the regimen in 39% of those enrolled and to the recommendation of its use only in those < age 65.[29]
These results suggest that the incremental benefit of minimal residual disease control with consolidative HDT-ASCT may not be present if R-hyperCVAD is used as induction, perhaps sparing patients the small but measurable risk of transplant-related mortality and more inpatient days compared with the other regimens. This is significant because hyperCVAD is associated with notable cumulative toxicity.[29] A counterpoint to the SWOG 0213 toxicity report is that in the NCCN series only one patient died of treatment-related complications despite the fact that more than 50% of patients received the studied induction regimens. This may reflect patient selection, better supportive measures, and/or greater familiarity with the regimens’ potential toxicities at centers included in this series than at those in the SWOG 0213 study. The NCCN study has many limitations, including the retrospective nature of the analysis; moreover, the number of hyperCVAD cycles received before HDT-ASCT likely varies at participating centers. Still, there are no data from European groups or others outside of the United States that suggest that an alternating or sequential cytarabine-containing induction approach without HDT-ASCT is inferior to induction plus consolidative HDT-ASCT.
A caveat about the NCCN analysis and a frequently encountered dilemma is that of the patient’s transplant status at the time of diagnosis of MCL. Often the transplant status is questionable, and patients may receive standard R-CHOP induction; their response and toleration of the regimen dictate whether they proceed to HDT-ASCT or maintenance rituximab therapy. In the hyperCVAD series from MD Anderson, patients had to have an Eastern Cooperative Oncology Group performance status of 0 or 1 before enrollment. This is a very attractive decision tree, given recent data demonstrating a survival advantage with maintenance rituximab therapy and the NCCN data. Currently, the NCCN guidelines recommend consolidation with HDT-ASCT in patients who obtain a CR or partial response to induction therapy.[30] Whether those patients who achieve a CR to induction therapy followed by maintenance rituximab therapy have similar PFS after consolidative HDT-ASCT is unknown.
Allogeneic Stem Cell Transplantation
In the pre-rituximab era, patients with relapsed or refractory MCL had poor remission durability with HDT-ASCT, even with a chemosensitive response. Currently, novel regimens incorporating bortezomib and/or bendamustine are efficacious in inducing a subsequent chemosensitive response and therefore provide an opportunity to consolidate with alloSCT. Because MCL is uncommon and occurs in older patients, no randomized clinical trials have compared ASCT and alloSCT in patients with relapsed and refractory MCL. Moreover, alloSCT data solely about patients with MCL are also uncommon because they are often included with other NHL subtypes in center-specific studies (Table 2).
A comparative analysis from UNMC retrospectively reviewed the outcomes of 97 patients who underwent either HDT-ASCT (n = 80) or myeloablative alloSCT (n = 17).[18] The patients in the alloSCT cohort were more heavily pretreated-which is inherent to alloSCT-but all were chemosensitive at the time of transplant. The relapse rate was higher in the HDT-ASCT cohort (56% vs 21%; P = .1), but transplant-related mortality, as expected, was higher in the alloSCT cohort.
In patients with relapsed and refractory MCL, consideration of alloSCT requires considerable planning and referrals; the work-up should parallel reinduction therapy in order not to miss the transplant window. Furthermore, reduced-intensity conditioning regimens likely have opened up this modality to many more patients than in the total body irradiation–based myeloablative era; however, this conditioning remains an option for younger persons. Nonetheless, alloSCT in a transplant-eligible patient with a human leukocyte antigen–identical donor remains a curative option. There are few, if any, scenarios in which alloSCT in patients with newly diagnosed MCL can routinely be recommended in the first chemosensitive response.
Conclusion
HDT-ASCT is recommended by the NCCN guidelines in transplant-eligible patients with newly diagnosed MCL who have a chemosensitive response to initial therapy. The induction chemotherapy regimens that were evaluated in the pre-rituximab era have stood the test of time, but less intense regimens such as rituximab plus bendamustine are attempting in clinical trials (eg, NCT01412879) to challenge the intensive multiagent regimen paradigm. Bendamustine has already demonstrated an impressive induction response rate and differing toxicity profiles in both the Study Group Indolent Lymphomas (StiL) and Bendamustine Hydrochloride and Rituximab Compared With R-CVP or R-CHOP in the First-Line Treatment of Patients With Advanced Indolent Non-Hodgkin’s Lymphoma or Mantle Cell Lymphoma (BRIGHT) studies compared with R-CHOP.[27,31] The role of HDT-ASCT in the relapsed and refractory setting is historical and has been replaced by alloSCT studies testing whether the risk is worth the potential reward of a long-term disease-free interval or cure.
Finally, the treatment of MCL has benefited from a wealth of agents through a committed pipeline that has resulted in US Food and Drug Administration approval and/or signs of efficacy of several novel agents in the relapsed and refractory setting. These novel agents-including the proteasome inhibitor bortezomib, the immunomodulatory agent lenalidomide, and a number of other oral compounds such as ibrutinib-are being targeted in up-front strategies to hopefully maintain efficacy, decrease patient length of stay, and reduce treatment-related toxicity. Outcomes in MCL have improved, but the relative contributions of rituximab, induction chemotherapy, consolidative transplant, and maintenance therapies remain unknown.
Financial Disclosure:Dr. Armitage serves as a consultant to GlaxoSmithKline, Seattle Genetics, Genentech, Roche, Spectrum Pharmaceuticals, and Ziopharm Oncology; he is also a member of the Board of Directors of Tesaro, Inc. Dr. Lunning has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article. The article was fully funded by Celgene.
References:
1. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909-18.
2. Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-76.
3. Swerdlow SH, Campo E, Harris NL, et al (editors). WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer Press; 2008.
4. Weisenburger DD, Sanger WG, Armitage JO, Purtilo DT. Intermediate lymphocytic lymphoma: immunophenotypic and cytogenetic findings. Blood. 1987;69:1617-21.
5. Yang WI, Zukerberg LR, Motokura T, et al. Cyclin D1 (Bcl-1, PRAD1) protein expression in low-grade B-cell lymphomas and reactive hyperplasia. Am J Pathol. 1994;145:86-96.
6. Williams ME, Nichols GE, Swerdlow SH, Stoler MH. In situ hybridization detection of cyclin D1 mRNA in centrocytic/mantle cell lymphoma. Ann Oncol. 1995;6:297-9.
7. Campo E, Raffeld M, Jaffe ES. Mantle-cell lymphoma. Semin Hematol. 1999;36:115-27.
8. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558-65.
9. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23:1984-92.
10. Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200-8.
11. Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687-93.
12. Le Gouill S, Callanan M, Macintyre E, et al. Clinical, metabolic and molecular responses after 4 courses of R-DHAP and after autologous stem cell transplantation for untreated mantle cell lymphoma patients included in the LyMa trial, a Lysa study [abstract 152]. Blood. 2012;120:21.
13. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520-31.
14. Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511-8.
15. Vose JM, Bierman PJ, Weisenburger DD, et al. Autologous hematopoietic stem cell transplantation for mantle cell lymphoma. Biol Blood Marrow Transplant. 2000;6:640-5.
16. Khouri IF, Romaguera J, Kantarjian H, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16:3803-9.
17. Freedman AS, Neuberg D, Gribben JG, et al. High-dose chemoradiotherapy and anti-B-cell monoclonal antibody-purged autologous bone marrow transplantation in mantle-cell lymphoma: no evidence for long-term remission. J Clinical Oncol. 1998;16:13-8.
18. Ganti AK, Bierman PJ, Lynch JC, et al. Hematopoietic stem cell transplantation in mantle cell lymphoma. Ann Oncol. 2005;16:618-24.
19. Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793-800.
20. Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677-84.
21. Dreyling M, Hoster E, Van Hoof A, et al. Early consolidation with myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission in mantle cell lymphoma: long term follow up of a randomized trial of the European MCL Network [abstract 285]. Blood. 2008;112:11.
22. Mangel J, Leitch HA, Connors JM, et al. Intensive chemotherapy and autologous stem-cell transplantation plus rituximab is superior to conventional chemotherapy for newly diagnosed advanced stage mantle-cell lymphoma: a matched pair analysis. Ann Oncol. 2004;15:283-90.
23. Geisler CH, Kolstad A, Laurell A, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158:355-62.
24. Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101-8.
25. Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48-53.
26. Hermine O, Hoster E, Walewski J, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) is superior to 6 courses CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: results of the MCL Younger Trial of the European Mantle Cell Lymphoma Network (MCL net) [abstract 151]. Blood. 2010;116:110.
27. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203-10.
28. LaCasce AS, Vandergrift JL, Rodriguez MA, et al. Comparative outcome of initial therapy for younger patients with mantle cell lymphoma: an analysis from the NCCN NHL Database. Blood. 2012;119:2093-9.
29. Bernstein SH, Epner E, Unger JM, et al. A phase II multicenter trial of hyperCVAD MTX/Ara-C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann Oncol. 2013;24:1587-93.
30. Zelenetz AD, Wierda WG, Abramson JS, et al. Non-Hodgkin’s lymphomas, version 3.2012. J Natl Compr Canc Netw. 2012;10:1487-98.
31. Flinn IW, Van der Jagt RH, Kahl B, et al. An open-label, random ized study of bendamustine and rituximab (BR) compared with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP) or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in first-line treatment of patients with advanced indolent non-Hodgkin’s lymphoma (NHL) or mantle cell lymphoma (MCL): the Bright study [abstract 902]. Blood. 2012;120:902.
32. Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535-42.
33. Cook G, Smith GM, Kirkland K, et al. Outcome following Reduced-Intensity Allogeneic Stem Cell Transplantation (RIC AlloSCT) for relapsed and refractory mantle cell lymphoma (MCL): a study of the British Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2010;16:1419-27.
34. Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144-52.
35. Le Gouill S, Kroger N, Dhedin N, et al. Reduced-intensity conditioning allogeneic stem cell transplantation for relapsed/refractory mantle cell lymphoma: a multicenter experience. Ann Oncol. 2012;23:2695-703.