Poor Risk Renal Cell Carcinoma Patients: Role of New Agents
Prognostic factor models can provide important information to help patients and clinicians make treatment decisions. These decisions have become more complex in the selection of treatment for patients with metastatic renal cell carcinoma (RCC).
Introduction
Prognostic factor models can provide important information to help patients and clinicians make treatment decisions. These decisions have become more complex in the selection of treatment for patients with metastatic renal cell carcinoma (RCC). Within the past 2 years, three new agents-sorafenib (Nexavar), sunitinib (Sutent), and temsirolimus (Torisel)-have gained approval for RCC, and it is likely that more will follow in the near future. In addition, the role of the older cytokine therapies, while clearly diminished, may still be important in selected patients.
Despite remarkable progress in the therapy of metastatic RCC, it is apparent that tumor characteristics and prognostic factors remain important determinants of disease control and survival. Patients with multiple adverse prognostic factors usually have been excluded from participation in clinical trials of cytokine therapy, and only a minority of such patients has been included in the recent trials of targeted agents. Selecting therapy for these poor risk or poor prognosis patients is an important clinical challenge.
Poor Risk Renal Cell Carcinoma
Several groups have developed prognostic factor models for patients with metastatic renal carcinoma. Despite differences in patient populations and the specific factors chosen for analysis, these models are remarkably similar in how they define good, intermediate, and poor prognosis patients.1-4
Adverse prognostic factors common to these models include low performance score, presence of anemia, elevated levels of serum lactate dehydrogenase (LDH) and calcium, less than 1 year’s time from diagnosis of RCC to the start of systemic treatment for metastatic disease, and more than one organ site (eg, lung, bone, liver) involved with metastatic disease. Patients with none of these poor prognostic factors have a median survival greater than 2 years, whereas those with three or more factors have a median survival of 4 to 8 months. In the Memorial Sloan-Kettering Cancer Center (MSKCC) renal cell carcinoma prognosis model, approximately 25% of patients had none of the factors predictive of shorter survival and so were considered “good risk,” while 53% of the population had one or two of these factors and were at “intermediate risk,” and 22% had three or more factors, which defined the poor prognosis group.1
How Temsirolimus Works
Temsirolimus (Torisel) is an inhibitor of the mammalian target of rapamycin (mTOR) kinase, a component of cell signaling pathways that sense energy balance, hypoxic stress, and growth stimuli. These signals are integrated by mTOR to regulate cell growth and proliferation, as well as angiogenesis.5-7
Temsirolimus and its active metabolite, sirolimus (Figure 1), bind to an abundant intracellular protein, FKBP-12.8 The drug-FKBP-12 complexes block mTOR signaling and consequently, the translation of proteins that are required for cell cycle progression and angiogenesis.9-11 This mechanism of action is relevant to RCC, a tumor that is characterized by unregulated angiogenesis.12 Consistent with this idea, encouraging antitumor activity was noted in a randomized phase II trial of temsirolimus for patients with RCC who had previously received cytokine therapy.13
Click to enlarge
The Global ARCC Study
The Global Advanced Renal Cell Carcinoma (ARCC) Trial was a multicenter randomized trial that compared three regimens as first-line treatment of patients with poor prognosis, advanced RCC (figure 2). The three treatment arms were: temsirolimus at 25 mg IV weekly; temsirolimus at 15 mg IV weekly, plus interferon at 6 million units (MU) SC three times a week (tiw); and single agent interferon, starting with a dose of 3MU tiw and escalating to a maximum of 18 MU tiw, as tolerated.
Click to enlarge
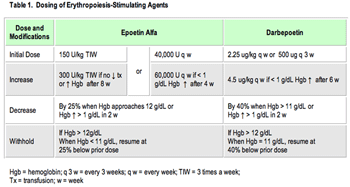
The primary efficacy endpoint for this study was overall survival, comparing each of the temsirolimus-containing groups with the single agent interferon group.15 The study was designed to detect a 40% improvement in median overall survival, with secondary endpoints of progression-free survival, objective response, and safety. A modification of the MSKCC prognostic model was used to enroll 626 poor prognosis patients (Table 1).
Click to enlarge
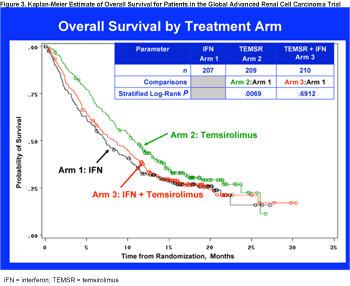
This trial demonstrated the superiority of temsirolimus over interferon in the poor prognosis population. The overall survival of patients receiving temsirolimus was significantly better than that of patients receiving single agent interferon (Figure 3).
Click to enlarge
Median survival times were 10.9 months for patients receiving temsirolimus, 7.3 months for patients receiving interferon, and 8.4 months for patients receiving the combination of temsirolimus and interferon (Table 2). Progression-free survival (PFS), determined by independent radiology assessement, was also significantly better for the temsirolimus group, 5.5 months vs 3.1 months for the interferon group (Table 2). The advantage of single agent temsirolimus vs interferon was also evident in the proportion of patients, 32.1% and 15.5%, respectively, with objective response or stable disease lasting for at least 24 weeks (Table 2). There were significantly fewer patients with serious adverse events in the temsirolimus group than in the interferon group (P = .02).
Click to enlarge
Interestingly, patients treated with the combination of temsirolimus and interferon did not survive longer than those receiving interferon alone, and the proportion of patients with severe toxicity was clearly greater in combination group. Therefore, the addition of interferon to temsirolimus is not recommended.
The toxicity profile of temsirolimus differs from that of the antivascular endothelial growth factor antibody bevacizumab (Avastin) and the multitargeted tyrosine kinase inhibitors sunitinib (Sutent) and sorafenib (Nexavar). The most common adverse events of temsirolimus are fatigue, rash, diarrhea, stomatitis, myelosuppression, hyperglycemia, and hyperlipidemia (Figure 4). The metabolic effects probably reflect inhibition of mTOR regulated glucose and lipid metabolism. Temsirolimus associated grade 3/4 hyperglycemia occurred in 11% of patients.15
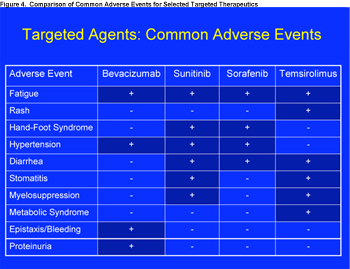
Click to enlarge
The key finding of the Global ARCC trial, that temsirolimus as first-line therapy improved overall survival of patients with poor prognosis renal carcinoma, led to the regulatory approval of this agent for the treatment of advanced RCC. As the only agent shown to improve overall survival, temsirolimus should be considered the current standard first-line therapy for poor prognosis RCC. The activity of temsirolimus with poor prognosis disease suggests that the drug may also benefit patients with a more favorable prognosis. Additional studies are needed to address this possibility.
Other Targeted Therapies
Two phase III randomized studies have established the efficacy of sunitinib and a combination of bevacizumab and interferon as first-line treatment of metastatic RCC in more favorable populations. Each of these studies included subgroups of patients with poor risk disease, according to the MSKCC prognostic factor model, and each had primary endpoints of PFS.
In the study reported by Motzer et al16 comparing sunitinib with interferon alfa (9MU TIW) and updated at the 2007 meeting of the American Society of Clinical Oncology,17 PFS was significantly better for patients assigned to sunitinib, and this difference applied across all prognostic groups (Table 3). In the poor prognosis subgroups (6% of the study population), the median PFS was 3.7 months for patients receiving sunitinib and 1.2 months for patients receiving interferon.
Click to enlarge
In the study reported by Escudier and colleagues at the 2007 meeting of the American Society of Clinical Oncology,18 the PFS of patients receiving bevacizumab plus interferon was significantly longer than that of patients receiving interferon alone. Median PFS was 10.2 months for the bevacizumab and interferon (9 MU tiw) group and 5.4 months for the interferon alone group. The difference in PFS was significant in patients with good and intermediate MSKCC prognosis, but bevacizumab had no apparent impact on PFS in the poor prognosis patients. The median PFS was 2.2 months and 2.1 months, respectively, for patients treated with and without bevacizumab (Table 4). It is not known whether sunitinib or the bevacizumab plus interferon combination will improve overall survival compared with interferon alone because the study populations have a more favorable prognosis. Consequently, longer follow-up will be required to reach the survival endpoint.
Click to enlarge
Summary and Future Research
It is clear from the clinical trial results described here that the survival of patients with poor prognosis RCC is short, with median survival under 1 year. Temsirolimus improves overall survival and PFS of this group, and is the recommended first-line therapy for poor risk RCC.
Conclusions regarding the role of bevacizumab and sunitinib are limited by the small number of poor prognosis patients included in the study populations, and by the lack of prospective studies to compare these agents and temsirolimus in any RCC population. Consistent with its effects in the broader RCC population, sunitinib improves PFS in poor prognosis patients. By contrast, a preliminary report suggests that the addition of bevacizumab to interferon does not improve PFS of poor risk patients.
Additional unanswered questions concern the optimal second-line therapy for the patient with poor risk RCC and which agent(s) should be preferred for metastatic RCC with non-clear cell histologies, such as papillary and chromophobe renal carcinoma. Patients with papillary RCC appear to be less responsive to sunitinib and sorafenib.19 Patients with non-clear cell tumors comprised approximately 20% of the Global ARRC study population and appeared to benefit from temsirolimus to an equal or greater extent compared with the larger group of patients with clear cell RCC.15 A prospective, comparative evaluation of temsirolimus, sunitinib, and other targeted agents in this population would be of greater value in determining optimal therapy for these patients.
Current and future clinical trials will also determine if combination therapy with two targeted agents is better than one. Inevitably, the prognostic factors and models for RCC will change as therapy improves, as our knowledge of RCC biology increases, and as factors that predict response to a given therapeutic agent are identified. Thus, the future definition of “poor risk” RCC will likely incorporate molecular markers in addition to clinical parameters that form the current definition.
CME Post-Test and Evaluation
Continuing Medical Education InformationPoor Risk Renal Cell Carcinoma Patients: Role of New Agents
Activity Release Date: August 1, 2007
Activity Expiration Date: August 1, 2008
About the Activity
This activity is based on a brief article developed as part of the E-Update Series and posted on the Web. The series is geared to oncologists and addresses new treatments of cancer or modifications thereof.
This activity has been developed and approved under the direction of Beam Institute.
Activity Learning Objectives
After reading this article, participants should be able to:
a. List adverse prognostic factors common to prognostic factor models for patients with metastatic renal cell carcinoma.
b. Correlate prognostic factors with expected median survival for patients with renal cell carcinoma.
c. Identify the mechanism of action of temsirolimus and why it is relevant to renal cell carcinoma.
d. List the key findings of the Global Advanced Renal Cell Carcinoma (ARCC) Trial.
e. Recognize that temsirolimus, as the only agent shown to improve overall survival for poor prognosis renal cell carcinoma, should be considered the current standard first-line therapy for poor prognosis patients.
f. Identify sunitinib and bevacizumab as other targeted agents used as first-line treatment of metastatic renal cell carcinoma and understand their impact on different prognostic groups.
g. Identify common adverse events associated with targeted agents used for treating renal cell carcinoma.
Target Audience
This activity targets physicians in the fields of oncology and hematology.
Accreditation
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Beam Institute and The Oncology Group. Beam Institute is accredited by the ACCME to provide continuing medical education for physicians
Continuing Education CreditAMA PRA Category 1 Credit™
The Beam Institute designates this educational activity for a maximum of 2 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Compliance Statement
This activity is an independent educational activity under the direction of Beam Institute. The activity was planned and implemented in accordance with the Essential Areas and policies of the ACCME, the Ethical Opinions/Guidelines of the AMA, the FDA, the OIG, and the PhRMA Code on Interactions with Healthcare Professionals, thus assuring the highest degree of independence, fair balance, scientific rigor, and objectivity.
However, Beam Institute, the Grantor, and CMPMedica shall in no way be liable for the currency of information or for any errors, omissions, or inaccuracies in the activity. Discussions concerning drugs, dosages, and procedures may reflect the clinical experience of the author(s) or may be derived from the professional literature or other sources and may suggest uses that are investigational in nature and not approved labeling or indications. Activity participants are encouraged to refer to primary references or full prescribing information resources. The opinions and recommendations presented herein are those of the author(s) and do not necessarily reflect the views of the provider or producer.
Financial Disclosures
Dr. Bukowski serves on the speakers' bureau for Bayer, Genentech, Pfizer, and Wyeth; is a consultant for Bayer, Genentech, Novartis, Pfizer, and Wyeth and receives research support from Pfizer and Wyeth. Dr. Motzer receives research support from Genentech, Pfizer, and Wyeth; and also serves on the speakers' bureau for Bayer. Dr. Hudes serves on the speakers’ bureau for Pfizer and is an advisory board member for Genentech, Pfizer and Wyeth.
Copyright
Copyrights owned by Beam Institute, a division of CME LLC. Copyright 2007, CME LLC. All rights reserved.
Contact Information
We would like to hear your comments regarding this or other activities provided by Beam Institute. In addition, suggestions for future activities are welcome. Contact us at:
Address:
Director of Continuing Education
Beam Institute
11 West 19th Street, 3rd Floor
New York, NY 10011-4280
Phone: 888-618-5781
Fax: 212-600-3050
e-mail:beaminstitute@cmp.com
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Motzer RJ, Bacik J, Murphy BA, et al: Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289-296, 2002.
2. Negrier S, Escudier B, Gomez F, et al: Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Français d’Immunotherapie. Ann Oncol (13):1460-1468, 2002.
3. Mekhail TM, Abou-Jawde RM, BouMerhi G, et al: Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 23:832-841, 2005.
4. Bukowski RM, Negrier S, Elson P: Prognostic factors in patients with advanced renal cell carcinoma: development of an international kidney cancer working group. Clin Cancer Res 10(suppl):6310s-6314s, 2004.
5. Wullschleger S, Loewith R, Hall MN: TOR signaling in growth and metabolism. Cell 124:471-484, 2006.
6. Fingar DC, Richardson CJ, Tee AR, et al: mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4EBP1/Eukaryotic Translation Factor 4E. Mol Cell Biol 24:200-216, 2004.
7. Hudson CC, Liu M, Chiang GG, et al: Regulation of hypoxia-inducible factor 1a expression and function by the mammalian target of rapamycin. Mol Cell Biol 22:7004-7014, 2002.
8. Skotnicki JS, Leone CL, Smith AL, et al: Design, synthesis and biological evaluation of C-42 hydroxyesters of rapamycin: The identification of CCI-779. Clin Cancer Res 7:3749S-3750S, 2001.
9. Yu K, Toral-Barza L, Discafani C, et al: mTOR, a novel target in breast cancer: The effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer 8:249-258, 2001.
10. Dudkin L, Dilling MB, Cheshire PJ, et al: Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res 7:1758-1764, 2001.
11. Thomas GV, Tran C, Mellinghoff IK, et al: Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nature Medicine 12:122-127, 2005.
12. Del Bufalo D, Ciuffreda L, Trisciuoglio D, et al: Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Clin Cancer Res 66:5549-5554, 2006.
13. Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA: Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res 9:4641-4652, 2003.
14. Atkins M, Hidalgo M, Stadler WM, et al: Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22:909-918, 2004.
15. Hudes G, Carducci M, Tomczak P, et al: Temsirolimus, interferon alfa, or both for advanced renal cell carcinoma. N Engl J Med 356:2271-2281, 2007.
16. Motzer RJ, Hutson TE, Tomczak P, et al: Sunitinib versus interferon alfa in metastatic renal cell carcinoma. N Engl J Med 356:115-124, 2007.
17. Motzer RJ, Figlin RA, Hutson TE, et al: Sunitinib versus interferon-alfa (IFN-α) as first-line treatment of metastatic renal cell carcinoma (mRCC): Updated results and analysis of prognostic factors (abstract 5024). J Clin Oncol 25(suppl 18), 2007.
18. Escudier B, Koralewski P, Pluzanska A, et al: A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-α2a vs placebo/interferon-α2a as first-line therapy in metastatic renal cell carcinoma (abstract 3). J Clin Oncol 25(suppl 18), 2007.
19. Plantade A, Choueiri T, Escudier B, et al: Treatment outcome for metastatic papillary and chromophobe renal cell carcinoma (RCC) patients treated with tyrosine-kinase inhibitors (TKIs) sunitinib and sorafenib (abstract 5037). J Clin Oncol 25(suppl 18), 2007.