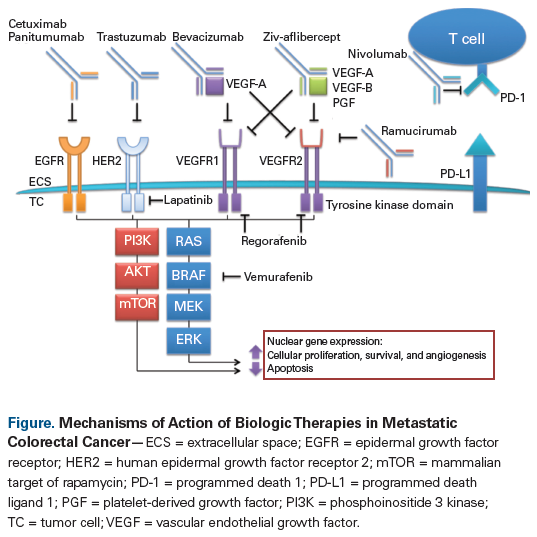
The survival of patients with metastatic colorectal cancer has improved dramatically in recent years, with overall survival exceeding 3 years in large randomized clinical trials. There are now several treatment options for patients with metastatic colorectal cancer. In addition to chemotherapy backbones utilizing fluoropyrimidine, oxaliplatin, and irinotecan combinations, biologic agents that target specific oncogenic pathways have contributed to the improved survival observed in this patient population. This class of medications includes epidermal growth factor receptor (EGFR)-targeted drugs (cetuximab and panitumumab) and vascular endothelial growth factor (VEGF)-targeted therapies (bevacizumab, ramucirumab, ziv-aflibercept, and regorafenib). Bevacizumab remains the only VEGF-targeted agent approved by the US Food and Drug Administration in the first-line metastatic setting. EGFR-directed treatment should be restricted to patients with extended RAS and BRAF wild-type tumors. Tumor sidedness may be a more powerful prognostic and predictive biomarker than tumor mutational profile. Patients with left-sided primary tumors derive greater benefit from EGFR-targeted therapies whereas patients with right-sided primary tumors benefit more from bevacizumab. Herein we review drugs that target the EGFR and VEGF pathways, focusing on patient selection, drug toxicities, and how to choose agents for first-line therapy.
Introduction
Colorectal cancer is a common malignancy and the second leading cause of cancer-related death in the United States.[1] Around one-fifth of patients have metastatic disease at the time of diagnosis, and their overall survival (OS) rate is only 13.5% at 5 years.[2] Nevertheless, the median OS of patients with metastatic colorectal cancer is currently 3 years, with results from large prospective clinical trials showing a continuing increase in survival rates.[3,4] Oncologists have an array of choices to consider in terms of possible combinations of cytotoxic and biologic drugs for patients with metastatic colorectal cancer.
For the purpose of this review, we define biologic therapies as pharmaceuticals that target specific carcinogenic pathways. Approved therapies include agents targeting the epidermal growth factor receptor (EGFR) and those targeting proteins essential to angiogenesis, including the vascular endothelial growth factor (VEGF) (Figure). Given the abundance of current therapeutic options for the treatment of colorectal cancer, there is often no single correct answer to the question of which therapies to select and how to sequence them. In this article, we will highlight the mechanisms of action of selected classes of biologic therapies, identify predictive biomarkers, and address how best to combine biologic agents with conventional cytotoxic chemotherapy.
The EGFR Pathway and Anti-EGFR Therapy
The ErbB family of receptor tyrosine kinases consists of the EGFR transmembrane glycoproteins Erbb1 (human epidermal growth factor receptor 1 [HER1]), HER2/neu (Erbb2), HER3 (Erbb3), and HER4 (Erbb4). EGFR is expressed in normal epithelial tissues and in several types of human cancers. Binding of EGF or other growth ligands to EGFR activates multiple downstream signal transduction pathways that promote cellular proliferation, including the RAS/RAF/MAPK/PI3K/AKT/mTOR pathways.[5] The anti-EGFR monoclonal antibodies cetuximab and panitumumab are approved by the US Food and Drug Administration (FDA) for use in patients with metastatic colorectal cancer who have KRAS wild-type tumors.[6,7]
Initially, EGFR expression was thought to be a positive predictive biomarker for response to anti- EGFR monoclonal antibodies, but work by Chung and colleagues published in 2005 demonstrated that EGFR-negative patients also have the potential to respond to cetuximab.[8] In subsequent studies by other investigators, published in 2008, KRAS mutations in exon 2 (codons 12 and 13), which are present in approximately 40% of patients with metastatic colorectal cancer, were found to confer de novo resistance to EGFR-directed therapies; this resistance is due to constitutive activation of the KRAS GTPase, which binds to and hydrolyzes guanosine triphosphate, bypassing the anti-EGFR effect on downstream signaling pathways.[9,10] Mutations in KRAS exons 3 and 4 and NRAS exons 2, 3, and 4 are also implicated in acquired resistance in patients treated with anti-EGFR monoclonal antibodies.[11,12] However, as far as predicting response to anti-EGFR therapy is concerned, extended RAS testing alone may not be sufficient to inform treatment decision making because mutations in PIK3CA (exon 20) and BRAF V600E have also been associated with low rates of response to anti-EGFR agents.[13] Therefore, the National Comprehensive Cancer Network (NCCN) recommends that all patients with metastatic colorectal cancer undergo tumor genotyping for KRAS, NRAS, and BRAF because anti-EGFR monoclonal antibodies should be administered only to patients with wild-type expression of these genes.[14]
Cetuximab
Cetuximab is a chimeric mouse/human immunoglobulin (Ig)G1 monoclonal antibody against EGFR. In the phase III CRYSTAL trial, the addition of cetuximab to FOLFIRI (leucovorin, fluorouracil [5-FU], and irinotecan) improved progression-free survival (PFS) compared with FOLFIRI alone.[15] Patients (N = 1,198) with previously untreated metastatic colorectal cancer that expressed EGFR by immunohistochemistry were randomized 1:1 to treatment with FOLFIRI plus cetuximab (at 400 mg/m2 IV over 120 minutes on day 1, then 250 mg/m2 weekly) or FOLFIRI alone. The primary endpoint of the study was median progression-free survival (PFS), which was shown to be longer with cetuximab (8.9 months vs 8.0 months; P = .048; Table), as was the objective response rate ORR (46.9% vs 38.7%; P = .004); however, median overall survival (OS) was not significantly different (19.9 months vs 18.6 months; P = .31). In a subsequent expanded analysis of 430 patients with tumors evaluable for extended RAS testing, adding cetuximab to the chemotherapy regimens of patients with RAS wild-type tumors led to significant improvements in median OS (28.4 months vs 20.2 months; P = .0024), as well as median PFS (11.4 vs 8.4 months; P < .001) and ORR (66.3% vs 38.6%; odds [OR], 3.11; 95% CI, 2.03–4.78; P < .001).[12] There was no corresponding improvement for patients with any RAS mutation. Cetuximab did cause more grade 3/4 adverse events, notably skin reactions (19.7% vs 0.2%), diarrhea (15.7% vs 10.5%), rash (8.2% vs 0%), and infusion reactions (2.5% vs 0%).[15]
In the phase II OPUS trial, 344 patients with previously untreated EGFR-positive metastatic colorectal cancer were randomized 1:1 to receive FOLFOX4 (oxaliplatin, leucovorin, and 5-FU) plus cetuximab (administered at the same dosing used in the previously described CRYSTAL trial) or FOLFOX4 alone.[16] The primary endpoint of the study was ORR, which was similar between treatment groups (46% [FOLFOX4 plus cetuximab] vs 36% [FOLFOX4 alone]; P = .064), but was significantly improved following FOLFOX4 plus cetuximab when patients with KRAS wild-type tumors only were singled out (61% with the combination vs 37% with FOLFOX4 alone; P = .011). The same pattern was seen for median PFS in patients with KRAS wild-type tumors only (7.7 months vs 7.2 months, respectively; P = .0163). However, in the KRAS-mutated population, median PFS was actually worse when cetuximab was incorporated into a FOLFOX4 regimen (5.5 months vs 8.6 months; P = .0192), indicating that RAS testing prior to treatment with an EGFR inhibitor is of the utmost importance. Among the most common grade 3/4 adverse events, rash was more common with cetuximab (11% with FOLFOX4 plus cetuximab vs 0.6% with FOLFOX4 alone), but rates of neutropenia (30% vs 34%) and diarrhea (8% vs 7%) were similar between the treatment groups.[16]
The phase III COIN trial again compared combination chemotherapy with a fluoropyrimidine and oxaliplatin, with or without cetuximab. In total, 1,630 patients with previously untreated metastatic colorectal cancer were randomized to receive chemotherapy (physician’s choice of capecitabine and oxaliplatin or FOLFOX4) plus cetuximab (at the standard approved dosing described previously) or chemotherapy alone.[17] Given the growing evidence that EGFR expression was not an effective biomarker for response to cetuximab, the revised primary endpoint was median OS in patients with KRAS wild-type tumors (729 patients). In this group, median OS was similar between treatment arms (17.0 months with chemotherapy plus cetuximab vs 17.9 months with chemotherapy alone; P = .67). In a subgroup of patients with tumors that expressed wild-type KRAS, as well as wild-type NRAS and BRAF, there was also no significant difference in median OS (19.9 months with chemotherapy plus cetuximab vs 20.1 months with chemotherapy alone; P = .86). Treatment that included cetuximab was associated with increased incidences of rash, nail changes, hand-foot syndrome, diarrhea, stomatitis, lethargy, anemia, and hypomagnesemia.
Similarly, the phase III NORDIC-VII trial randomized 571 patients with previously untreated metastatic colorectal cancer in a 1:1 ratio to receive the FLOX regimen (bolus 5-FU, leucovorin, and oxaliplatin) alone or with cetuximab.[18] There was no significant survival advantage following the addition of cetuximab to FLOX: median PFS (the primary endpoint) was 8.3 months in patients treated with FLOX and cetuximab vs 7.9 months in patients treated only with FLOX (P = .31), and median OS was 20.4 months vs 19.7 months, respectively (P = .67). ORR also remained essentially unchanged (49% vs 41%, respectively; P = .15). In the KRAS wild-type population, there was still no median OS benefit from the addition of cetuximab (22.0 months with FLOX and cetuximab vs 20.1 months with FLOX alone; P = .48). Therefore, cetuximab did not provide a survival benefit when added to the FLOX regimen.
Given the discordant findings between the CRYSTAL trial (which demonstrated improved median OS with the addition of cetuximab to FOLFIRI in RAS wild-type patients) and the COIN, OPUS, and NORDIC-VII trials (which did not demonstrate a survival benefit in this setting), it was concluded that cetuximab has the greatest efficacy in RAS wild-type patients when combined with irinotecan-based rather than oxaliplatin-based chemotherapy.[19] Confirmation of this theory requires further study of cetuximab administered together with irinotecan-based regimens as first-line treatment of patients with RAS wild-type metastatic colorectal cancer.
Panitumumab
The other FDA-approved anti-EGFR agent in metastatic colorectal cancer is panitumumab, a fully humanized IgG2 monoclonal antibody. The landmark phase III PRIME study randomized 1,183 patients with untreated metastatic colorectal cancer to receive FOLFOX4 plus panitumumab (with the latter administered at a dosage of 6 mg/kg IV over 1 hour, then 6 mg/kg over a 30-minute period every 2 weeks thereafter) or FOLFOX4 alone.[20] The primary endpoint was PFS, and patients were analyzed according to KRAS mutational status in a prospective manner. In the KRAS wild-type population, median PFS was significantly longer in patients treated with the panitumumab/chemotherapy combination (9.6 months vs 8.0 months with FOLFOX4 alone; P = .02). Although there was a trend toward increased median OS following chemotherapy plus panitumumab, this did not reach statistical significance (23.9 months vs 19.7 months with chemotherapy alone; P = .072). Conversely, median PFS was significantly shorter following administration of panitumumab to patients with KRAS-mutated tumors (7.3 months vs 8.8 months; P = .02) and, although median OS followed a trend similar to that of median PFS, there was no significant difference between treatment groups (median OS, 15.5 months with chemotherapy plus panitumumab vs 19.3 months with chemotherapy alone; P = .068).[20] Skin toxicity was common, affecting 96% of patients who received panitumumab, and 34% of patients had grade 3/4 skin toxicity. Only 2 patients (0.3%) had grade 3 infusion reactions; both subsequently received premedication therapy, enabling them to complete additional cycles of treatment with panitumumab.
In an updated analysis of the PRIME study, evaluating RAS and BRAF mutations, patients without RAS or BRAF mutations had significantly longer median PFS (10.8 months vs 9.2 months; P = .002) and median OS (28.3 months vs 20.9 months; P = .02) with panitumumab.[21] Patients with mutations of RAS or BRAF had inferior median PFS (7.3 months vs 8.0 months; P = .03) and median OS (15.3 months vs 18.0 months; P = .06) with the addition of panitumumab to their chemotherapy regimen. Therefore, administration of panitumumab in conjunction with FOLFOX4 is an effective first-line treatment option for patients with RAS and BRAF wild-type metastatic colorectal cancer only. Use of this combination should be avoided in patients whose tumors have RAS and BRAF mutations.
The bottom line: anti-EGFR therapy improves outcomes in metastatic colorectal cancer
Overall, we conclude that the addition of EGFR inhibitors to chemotherapy in the first-line setting in patients with RAS and BRAF wild-type metastatic colorectal cancer improves patient survival and disease outcomes. Nevertheless, because these agents yield only marginal improvements in OS, treatments with greater efficacy are needed.
The VEGF Pathway and Anti-VEGF Therapy
Vascular endothelial growth factor A (VEGF-A) promotes endothelial cell proliferation and angiogenesis by binding to cell surface receptors (VEGFRs). Blocking the interaction between VEGF and VEGFRs inhibits growth of the microvasculature and impedes progression to metastatic disease.[22]
Bevacizumab
Bevacizumab, a recombinant humanized monoclonal antibody against VEGF-A, is FDA-approved for the treatment of multiple cancer types (including metastatic colorectal cancer, nonsquamous non–small-cell lung cancer [NSCLC], glioblastoma, metastatic renal cell carcinoma, cervical cancer, and platinum-resistant ovarian cancers).[23] Aside from its direct antiangiogenic effects, bevacizumab may also normalize tumor vasculature, thereby improving the delivery of cytotoxic chemotherapy to tumor tissue.[24]
Hurwitz and colleagues demonstrated that adding bevacizumab to combination treatment with the IFL regimen (irinotecan, bolus 5-FU, and leucovorin) improved survival in patients with metastatic colorectal cancer.[25] In their phase III trial, 813 patients with untreated metastatic colorectal cancer were randomized in a 1:1 ratio to IFL plus bevacizumab (with the latter administered at a dosage of 5 mg/kg IV every 2 weeks) or IFL plus placebo. OS, the primary study endpoint, was found to be significantly improved following therapy with bevacizumab (median OS, 20.3 months vs 15.6 months with placebo; P < .001). Significant improvement in median PFS was also seen following treatment with bevacizumab (10.6 months with IFL and bevacizumab vs 6.2 months with placebo; P < .001), as was ORR (44.8% vs 34.8%, respectively; P = .004). Patients who received bevacizumab were more likely to have grade 3/4 adverse events (84.9% vs 74.0% with placebo; P < .01)-notably, hypertension (11.0% vs 2.3% with placebo; P < .01) and, rarely, gastrointestinal perforation (1.5% vs 0% with placebo; P < .01). This study firmly established the combination of bevacizumab with cytotoxic chemotherapy as the standard of care in first-line treatment of metastatic colorectal cancer. Notably, in the large phase III TRIBE trial, which demonstrated an OS benefit with frontline treatment with FOLFOXIRI (leucovorin, 5-FU, oxaliplatin, and irinotecan) compared with FOLFIRI, patients in both treatment arms received bevacizumab.[4,26] Results from this study showed that bevacizumab combined with irinotecan-based chemotherapy is an effective first-line treatment for patients with metastatic colorectal cancer, despite increased toxicities.
In a similar study, the phase III N01966 trial, 1,401 patients with untreated metastatic colorectal cancer were randomized in a 2 × 2 factorial fashion to receive combination treatment with CAPOX (capecitabine and oxaliplatin) or FOLFOX4, plus placebo or bevacizumab.[27] Patients who received bevacizumab had a significantly improved median PFS compared with those receiving placebo (9.4 months vs 8.0 months; P = .0023); however, there was no statistically significant improvement in median OS (21.3 months vs 19.9 months; P = .077) or ORR (47% vs 49%; P = .31). Nevertheless, bevacizumab combined with oxaliplatin and a fluoropyrimidine in the first-line setting is an appropriate treatment option.
In the phase III AVEX study, 280 patients (≤ 70 years of age) with untreated metastatic colorectal cancer were randomized in a 1:1 ratio to receive capecitabine plus bevacizumab or capecitabine alone.[28] PFS, the primary study endpoint, was found to be significantly improved in patients receiving bevacizumab (9.1 months) compared with those receiving chemotherapy alone (5.1 months; P < .0001). This was also the case for ORR (19% vs 10%, respectively; P = .04), although there was only a trend toward longer median OS (20.7 vs 16.8 months; P = .18). There were higher rates of grades 3 to 5 toxicities with bevacizumab, including venous thromboembolic events (8% vs 4%) and hand-foot syndrome (16% vs 7%). Although no OS benefit was demonstrated, given the significant PFS benefits reported, combination treatment with bevacizumab plus capecitabine is a reasonable option in older patients who have previously untreated metastatic colorectal cancer.
Ziv-aflibercept
The antiangiogenic agent ziv-aflibercept traps the ligands VEGF-A, VEGF-B, and placental growth factor, preventing their interaction with VEGFR-1 and VEGFR-2[29] In the first-line setting, ziv-aflibercept plus modified FOLFOX6 (mFOLFOX6) did not improve survival when compared with mFOLFOX6 alone. The phase II AFFIRM trial randomized 236 patients with untreated metastatic colorectal cancer in a 1:1 ratio to receive mFOLFOX6 plus ziv-aflibercept or mFOLFOX6 alone. There was no difference in median PFS (the primary endpoint) for patients receiving the combination therapy (8.48 months ) compared with those receiving mFOLFOX6 alone (8.77 months; P > .05). The same was the case for ORR (49.1% vs 45.9%, respectively; P > .05).[30] A trial of first-line treatment with FOLFIRI with or without ziv-aflibercept (the MINOAS trial) is underway in Greece (ClinicalTrials.gov identifier: NCT02624726).
Ramucirumab
Ramucirumab is a fully humanized IgG1 monoclonal antibody that specifically blocks the ligand-binding site of VEGFR-2. It is FDA-approved for use in platinum-resistant NSCLC and gastric and gastroesophageal adenocarcinoma.[31] An open-label, single-arm phase II study of ramucirumab in combination with mFOLFOX6 chemotherapy in 48 patients with untreated metastatic colorectal cancer demonstrated a median PFS of 11.5 months, a median OS of 20.4 months, and an ORR of 58.3%. Adverse events included grade 3/4 neutropenia (41.6%), hypertension (16.7%), and neuropathy (12.5%).[32] No randomized phase III data on ramucirumab in the first-line treatment of metastatic colorectal cancer have been published, and this agent is not FDA-approved for use in the first-line setting.
Combination Biologic Therapy
The combination of anti-EGFR and anti-VEGF biologic therapies should be avoided. The phase IIIB PACCE trial randomized 1,053 patients with untreated metastatic colorectal cancer in a 1:1 ratio to receive chemotherapy (a fluoropyrimidine plus oxaliplatin or irinotecan) with bevacizumab and panitumumab or chemotherapy plus bevacizumab only.[33] The addition of panitumumab increased toxicity and actually decreased median PFS (oxaliplatin cohorts: 10.0 months vs 11.4 months; irinotecan cohorts: 10.1 months vs 11.7 months;) and median OS (oxaliplatin cohorts: 19.4 months vs 24.5 months; irinotecan cohorts: 20.7 months vs 20.5 months). These findings persisted in the KRAS wild-type subpopulation. Patients receiving panitumumab also had a lower relative dose intensity of bevacizumab and chemotherapy than those who did not receive panitumumab, likely contributing to the survival outcomes. Similarly, the phase III CAIRO2 trial randomized 755 patients with untreated metastatic colorectal cancer in a 1:1 ratio to CAPOX plus bevacizumab and cetuximab or CAPOX plus bevacizumab alone (with PFS as the primary endpoint).[34] Patients who received both biologic therapies had inferior median PFS (9.4 months vs 10.7 months with bevacizumab alone; P = .01) and a trend toward inferior median OS (19.4 months vs 20.3 months, respectively; P = .16). ORR was similar between the two arms (52.7% vs 50.0%; P = .49). Within the group of patients who received dual biologic therapy, those with KRAS wild-type tumors fared better than those with KRAS-mutated tumors (median OS, 21.8 months vs 17.2 months, respectively; P = .06), although the study data do not support the use of both biologic therapies even in KRAS wild-type patients (in whom median OS was 22.4 months with bevacizumab vs 21.8 months with cetuximab and bevacizumab; P = .64).
Neoadjuvant Therapy in Resectable Metastatic Colorectal Cancer With Liver Metastases
Can biologic agents be added to chemotherapy in order to increase the chance of rendering liver metastases resectable? Starting with cetuximab in this setting, evidence of benefit is lacking. The phase III New EPOC trial randomized 272 patients with KRAS exon 2 wild-type metastatic colorectal cancer and potentially resectable liver metastases to chemotherapy (FOLFOX, CAPOX, or FOLFIRI) plus cetuximab or chemotherapy alone in a 1:1 ratio.[35] Patients who received cetuximab actually had inferior median PFS (14.1 months vs 20.5 months; P = .030), with a trend toward inferior median OS (39.1 months vs not reached at point of follow-up; P = .16) and similar liver resection rates (87% vs 93%), although this study should possibly take place in pan-RAS/BRAF wild-type patients, and perhaps in patients with only left-sided tumors (see section on tumor sidedness in this article). There was a trend toward improved PFS with cetuximab in patients who received FOLFIRI, but there were only 26 patients in this group. Regardless, neoadjuvant cetuximab is not recommended in this setting.
The phase II OLIVIA trial validated the use of neoadjuvant bevacizumab for patients with initially unresectable liver metastases. Eighty patients were randomized in a 1:1 ratio to treatment with FOLFOXIRI plus bevacizumab or mFOLFOX6 plus bevacizumab.[36] The resection rate was higher following therapy with FOLFOXIRI plus bevacizumab (61% vs 49%), as were the rate of R0 resection (49% vs 23%), ORR (81% vs 62%), and median PFS (18.6 months vs 11.5 months). Although this study did not compare chemotherapy plus bevacizumab against chemotherapy alone and thus cannot address the contribution made by adding bevacizumab to chemotherapy in this setting, these findings support the safety of bevacizumab plus chemotherapy in the neoadjuvant setting prior to liver resection.
Selection of Biologic Therapies in the First Line
Given the panoply of biologic therapies from which to choose, how does the treating oncologist select which agent and which chemotherapy backbone to use? As discussed, EGFR-directed therapies should be restricted to patients with RAS and BRAF wild-type tumors, and not all biologic therapies are approved for the first-line treatment of patients with metastatic colorectal cancer. For patients with RAS-mutated tumors, bevacizumab remains the biologic agent of choice in the first-line setting, and can be administered in combination with capecitabine, CAPOX, FOLFOX, FOLFIRI, or FOLFOXIRI.[3,28,34,37]
For patients with RAS wild-type tumors, choosing the most appropriate first-line treatment regimen is more complicated. Is it better to use a VEGF-targeting agent or an EGFR-targeting agent in the first-line treatment of patients with RAS wild-type tumors? In the phase III German Arbeitsgemeinschaft Internistische Onkologie FIRE-3 trial, 592 patients with untreated KRAS exon 2 wild-type metastatic colorectal cancer were randomized 1:1 to FOLFIRI plus cetuximab or FOLFIRI plus bevacizumab.[38] Adverse events were as expected, including more grade 3/4 skin reactions with cetuximab (26% vs 2% with bevacizumab). The primary endpoint of ORR was very similar between the groups (62% vs 58%; P = .18), as was median PFS (10.0 months vs 10.3 months; P = .55). However, median OS was greater following cetuximab than following bevacizumab therapy (28.7 months vs 25.0 months; P = .017). In a subgroup analysis of patients with RAS wild-type tumors, median OS favored the cetuximab arm (33.1 months vs 25.0 months; P = .0059), although median PFS (10.3 months vs 10.2 months; P = .77) and ORR (65.3% vs 58.7%; P = .18) were similar between the treatment arms.[39] Radiologic review showed that cetuximab was superior to bevacizumab in terms of the frequency of early tumor shrinkage (68.2% vs 49.1%, respectively; P = .0005) and median depth of response (–48.9% vs –32.3%; P < .0001),[39] indicating again that RAS wild-type tumors are particularly responsive to first-line FOLFIRI plus cetuximab.[39,40]
KEY POINTS
- All patients with metastatic colorectal cancer should undergo testing for KRAS, NRAS, and BRAF status and microsatellite instability/mismatch repair status.
- The addition of epidermal growth factor receptor inhibitors to chemotherapy in the first-line setting in patients with RAS and BRAF wild-type metastatic colorectal cancer improves survival.
- Patients with right-sided primary colon tumors have worse overall survival and derive less benefit from cetuximab (even if their tumors are KRAS wild-type) than patients with left-sided tumors.
- While great strides have been made, many patients still do not reap an overall survival benefit from currently available biologic therapy. Therefore, additional novel therapies and combination approaches are needed.
In contrast, the Cancer and Leukemia Group B (CALGB)/Southwest Oncology Group (SWOG) 80405 study showed no difference in median OS between cetuximab and bevacizumab in the first-line setting.[41] In this phase III study, 1,137 patients with untreated KRAS exon 2 wild-type metastatic colorectal cancer were randomized in a 1:1 ratio to cetuximab or bevacizumab in combination with either FOLFIRI or mFOLFOX6 (patient’s and physician’s choice, with 73.4% deciding on treatment with mFOLFOX6). The primary endpoint, median OS, was the same between the treatment groups (29.0 months for both cetuximab and bevacizumab; P = .34), as was median PFS (10.45 months vs 10.84 months). As noted, the majority of patients received oxaliplatin (not irinotecan), and EGFR-targeting agents may be more effective in combination with irinotecan than with oxaliplatin. Nevertheless, both regimens are reasonable choices in the first-line setting.
The phase II PEAK study randomized 285 patients with KRAS exon 2 wild-type untreated metastatic colorectal cancer to mFOLFOX6 plus panitumumab or mFOLFOX6 plus bevacizumab.[42] The primary endpoint of median PFS was not significantly improved with panitumumab (10.9 months vs 10.1 months; P = .353), but median OS was significantly improved (34.2 months vs 24.3 months; P = .009). OS may have been confounded by the effects of subsequent treatment regimens (since most patients subsequently received anti-EGFR and/or anti-VEGF agents). However, given the demonstrated improvements in median OS, mFOLFOX6 plus panitumumab is an appropriate first-line treatment for patients with metastatic colorectal cancer.
Tumor Sidedness as a Prognostic and Predictive Marker
The right side of the colon (extending from the cecum to the proximal two-thirds of the transverse colon) and the left side of the colon (incorporating the distal transverse to sigmoid colon and rectum) arise from different embryological origins (midgut and hindgut, respectively) and have different dominant blood supplies (superior mesenteric and inferior mesenteric arteries, respectively). Results from the FIRE-3, CRYSTAL, CALGB/SWOG 80405, and National Cancer Institute of Canada CO.17 trials, as well as Surveillance, Epidemiology, and End Results program database analyses, demonstrate that patients with right-sided tumors have worse OS and derive less benefit from cetuximab (even if their tumors are KRAS wild-type) than patients with left-sided tumors.[43-46]
For example, patients in the CALGB/SWOG 80405 study with KRAS wild-type left-sided tumors had a median OS of 37.5 months with cetuximab and 32.1 months with bevacizumab, whereas patients with KRAS wild-type right-sided tumors had a median OS of 16.4 months with cetuximab and 24.5 months with bevacizumab (hazard ratio [HR] for cetuximab 1.97; P < .0001; HR for bevacizumab 1.26; P < .0001).[45] These findings suggest that anti-EGFR therapies should be avoided in patients with right-sided tumors, and that bevacizumab should be used instead. The underlying biological differences between right colon, left colon, and rectal tumors are likely the cause of these differences in response, but more research is necessary to better characterize the differences.[47] Although these trials were conducted in the first-line metastatic setting, it may be possible to extrapolate their findings regarding tumor sidedness to later lines of therapy. At a minimum, these study results should be prospectively validated, and future trials in metastatic colorectal cancer should stratify patients based on tumor sidedness.
Conclusion
In our practice, we favor the use of mFOLFOX6 or CAPOX plus bevacizumab in the first-line RAS wild-type setting, saving irinotecan and EGFR-directed therapy for later lines of treatment. We also favor using bevacizumab for patients with right-sided colon primaries and anti-EGFR therapies for patients with left-sided primaries.
Biologic therapies remain integral to the treatment of patients with metastatic colorectal cancer. We are now better able to tailor these therapies to individual patients and thus optimize their treatment response. Bevacizumab is the most versatile biologic anticancer agent because it is effective regardless of RAS mutational status. Anti-EGFR therapy should be used in patients with RAS wild-type tumors and may be reserved for later lines of therapy. Combination biologic therapy should be avoided outside the context of a clinical trial. While great strides have been made, many patients still do not experience an OS benefit; additional novel therapies and combination approaches are needed.
Financial Disclosure:Dr. Salem serves as a speaker for, and consultant to, Genentech. The other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. American Cancer Society. Cancer facts & figures 2016. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures-2016.html Accessed June 23, 2017.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colon and rectum cancer. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed June 7, 2017.
3. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306-15.
4. Cremolini C, Loupakis F, Antoniotti C, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26:1188-94.
5. Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21-6.
6. US Food and Drug Administration. Highlights of prescribing information. Erbitux. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125084s0228lbl.pdf. Revised January 2012. Accessed June 7, 2017.
7. US Food and Drug Administration. Highlights of prescribing information. Vectibix. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125147s080lbl.pdf. Revised July 2009. Accessed June 7, 2017.
8. Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803-10.
9. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-65.
10. Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-34.
11. Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532-6.
12. Van Cutsem E, Lenz HJ, Kohne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692-700.
13. De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-62.
14. National Comprehensive Cancer Network. NCCN guidelines. Colon cancer. Version 1.2017. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed June 7, 2017.
15. Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-17.
16. Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-71.
17. Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-14.
18. Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-62.
19. Grothey A, Lenz HJ. Explaining the unexplainable: EGFR antibodies in colorectal cancer. J Clin Oncol. 2012;30:1735-7.
20. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-705.
21. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-34.
22. Arii S, Mori A, Uchida S, et al. Implication of vascular endothelial growth factor in the development and metastasis of human cancers. Hum Cell. 1999;12:25-30.
23. US Food and Drug Administration. Highlights of prescribing information. Avastin. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125085s225lbl.pdf. Revised September 2011. Accessed June 7, 2017.
24. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987-9.
25. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-42.
26. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-18.
27. Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-9.
28. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077-85.
29. Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393-8.
30. Folprecht G, Pericay C, Saunders MP, et al. Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: the AFFIRM study. Ann Oncol. 2016;27:1273-9.
31. US Food and Drug Administration. Highlights of prescribing information. Cyramza. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125477s007lbl.pdf. Revised December 2014. Accessed June 7, 2017.
32. Garcia-Carbonero R, Rivera F, Maurel J, et al. An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer. Oncologist. 2014;19:350-1.
33. Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-80.
34. Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-72.
35. Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601-11.
36. Gruenberger T, Bridgewater J, Chau I, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702-8.
37. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-44.
38. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-75.
39. Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426-34.
40. Modest DP, Stintzing S, Fischer von Weikersthal L, et al. Relation of early tumor shrinkage (ETS) observed in first-line treatment to efficacy parameters of subsequent treatment in FIRE-3 (AIOKRK0306). Int J Cancer. 2017;140:1918-25.
41. Venook AP, Niedzwiecki D, Lenz H, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(suppl):abstr LBA3.
42. Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240-7.
43. Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2016 Oct 10. [Epub ahead of print]
44. Brule SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51:1405-14.
45. Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary tumor location on overall survival and progression-free survival in patients with metastatic colorectal cancer: analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(15 suppl) :abstr 3504.
46. Schrag D, Weng S, Brooks G, et al. The relationship betwen primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol. 2016;34 (15 suppl) :abstr 3505.
47. Marshall J, Lenz HJ, Xiu J, et al. Molecular variances between rectal and left-sided colon cancers. J Clin Oncol. 2017;35:abstr 522.
48. Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37.
49. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-506.
50. Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre phase 3 study. Lancet Oncol. 2015;16:499-508.
51. Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-8.
52. Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311-9.
53. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346-55.
54. Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-13.
55. Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749-59.
56. Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-64.
57. Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569-79.
58. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-12.
59. Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-46.
60. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-20.