The Painful Problem of Biosimilars in the Clinic
Co-editor-in-Chief Howard S. Hochster, MD, discusses persistent obstacles to the effective use of biosimilars in clinical practice.

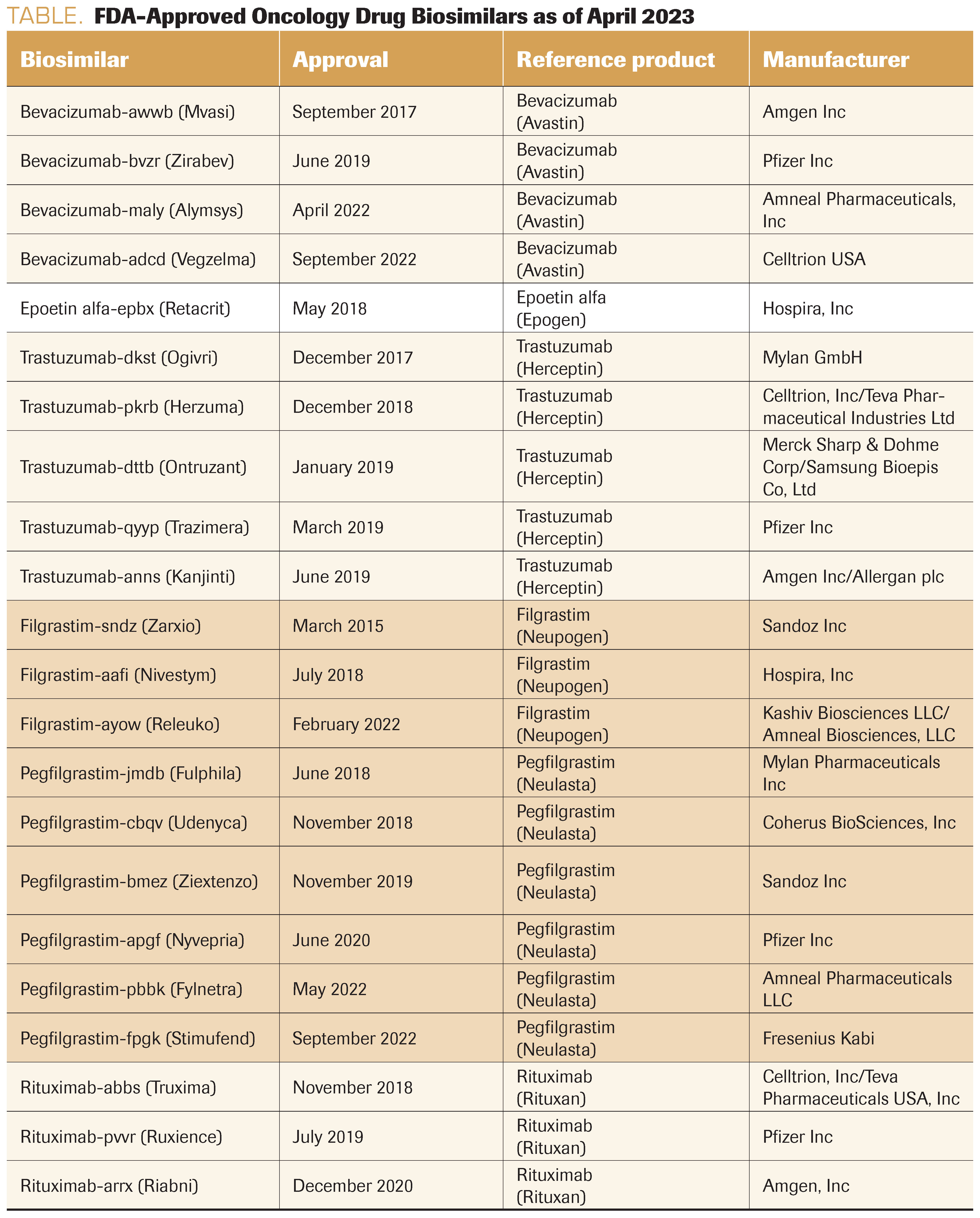
TABLE. FDA-Approved Oncology Drug Biosimilars as of April 2023
Just this week I ordered erythropoietin for a patient with chemotherapy-induced anemia. It was obvious, and the treatment was completely indicated. However, my electronic medical records order set had it listed as epoetin alfa (Procrit), so it took many hours and emails to eventually get epoetin alfa-epbx (Retacrit) approved by his insurance.
Why the major hassle for the use of biosimilar drugs (Table)? Why can’t we just order erythropoietin, filgrastim (Neupogen), bevacizumab (Avastin), or trastuzumab (Herceptin) and receive approval for any of the biosimilars?
The approval process for biosimilars is a result of the Biologics Price Competition and Innovation Act of 2009 (BPCI Act), a part of Obamacare. This congressional act was meant to parallel the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Amendments, for generic drugs, but sadly, the FDA has inadvertently undermined this process and created a lot of pain in the clinic.
The Hatch-Waxman Amendments created an important pathway for generic drugs, which allowed compounds of an identical chemical structure and bioequivalence to be approved without clinical trials. However, with the advent of “biologic” compounds that are not synthesized, but rather harvested from cell cultures, the drugs would never be chemically identical. Note: Even original brand compounds are not identical to themselves after a few years due to biological “drift”!
Therefore, the BPCI Act created an alternative pathway for biologically manufactured macromolecules to be considered “biosimilar.” If the drug acts on the same target and is considered “highly similar with no meaningful clinical differences,” it can be approved as a biosimilar. The preponderance of data required is preclinical, with laboratory analyses and binding assays, for example. In addition, one clinical trial is required to demonstrate the equivalent clinical activity to the originator molecule. If the agent is approved based on 1 trial, the biosimilar receives all the FDA-approved use indications that apply to the originator.
Then there is the problem of further FDA regulation. The FDA has legitimate concerns about monitoring the clinical activity and toxicity of these biosimilars through the “pharmacovigilance” program. To better track these varied biosimilars, the FDA issued a guidance in 2017 requiring all biologics to append the generic name with a 4-letter suffix, which should be random and of no meaning. This would allow better tracking of any problems with a particular biosimilar from a certain manufacturer.
However, by requiring the suffix, the FDA created a usage nightmare. Each biosimilar has its own identity today as a “branded biosimilar” (eg, for bevacizumab, Avastin is the originator and bevacizumab-awwb [Mvasi], bevacizumab-bvzr [Zirabev], and bevacizumab-maly [Alymsys] are all biosimilars). In addition, each of these drugs has its own J-code for billing. As a result, it becomes a game of ordering a biosimilar, insurance denying, and then trying to guess which one they will cover for payment. We have to reorder the drug with the correct biosimilar for which the insurer will pay. This is undesirable in a number of ways: (1) causes confusion in ordering the proper biosimilar, (2) is a waste of time for approvals and correction of orders, (3) requires additional pharmacy space and effort to stock multiple biosimilars because we cannot predict which one will be reimbursed, and (4) results in a lack of real competition between biosimilars, resulting in less competition and price reduction.
The need for pharmacovigilance should be balanced against the downstream pain experienced every day in the clinic. The drugs can be traced by manufacturer and lot number without suffixes and branded biosimilars (as with generic drugs). This is a case of “belt and suspenders,” and the belt is way too tight!
We should also note that clinicians have no scientific basis for preferring 1 approved biosimilar over another. We do not see the data on approvals and have to look hard to even find the manufacturer. To date, no biosimilar product has failed to live up to expectations as a drug compared with the originator drug, yet we experience the pain of prescribing biosimilars daily in the clinic.

How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.