Prognostic and Predictive Markers for the New Immunotherapies
Characterizing tumors by PD-L1 expression, immune infiltration, chemokine signature, and tumor mutational frequency may be a means of creating an integrated model for determining which patients may benefit from which immune-checkpoint inhibitors, either alone or in combination.
Figure 1: Two Distinct Mechanisms of Immune Resistance
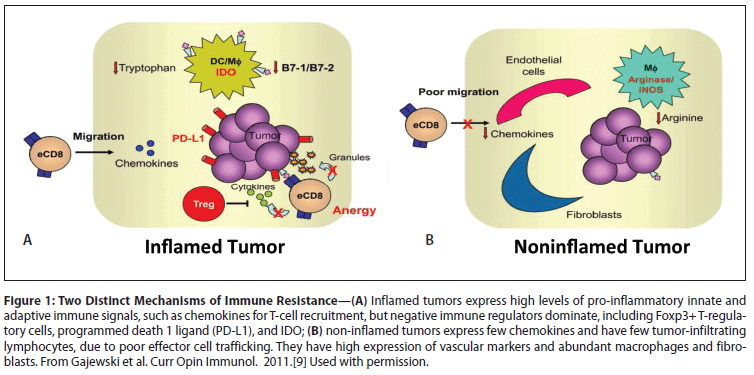
Figure 2: Expression of Programmed Death 1 Ligand (PD-L1) in Kidney Cancer and Normal Kidney
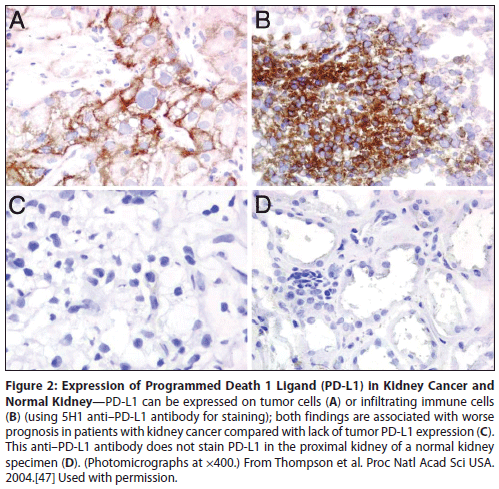
Figure 3: Intratumoral PD-L1 Expression Is Associated With Increased Clinical Benefit Across PD-1/PD-L1–Directed Therapy With Low Specificity
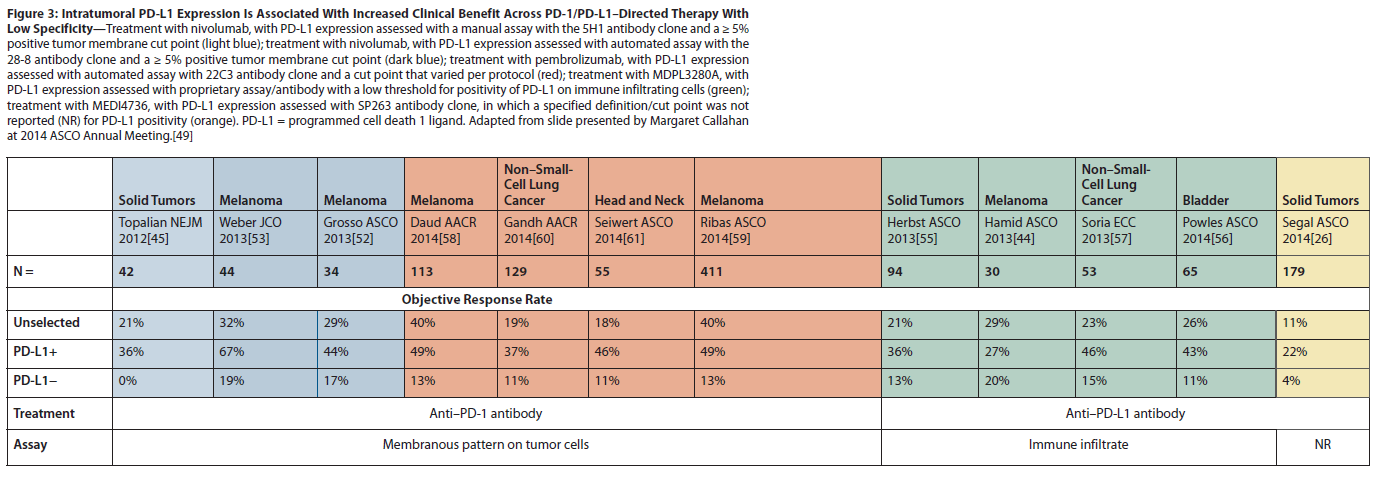
Figure 4: PD-L1 Expression and Intratumoral Inflammation in Tumors Are Two Key Variables in Tumors, Which May Be Central to Distinguishing Tumors That Are More Likely to Be Responsive to Immunotherapy, and May Suggest the Mechanism of an Individual’s Tumorigenesis
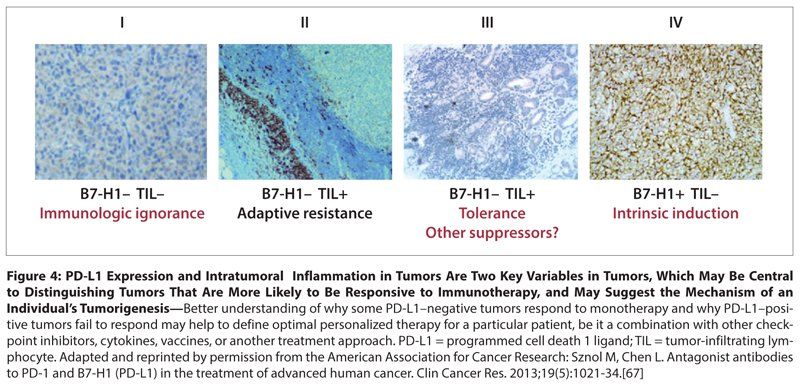
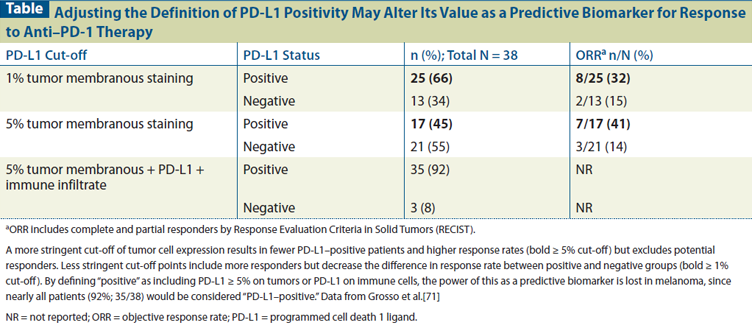
Table: Adjusting the Definition of PD-L1 Positivity May Alter Its Value as a Predictive Biomarker for Response to Anti–PD-1 Therapy
Blocking the programmed cell death 1 (PD-1) pathway with monoclonal antibodies has shown promising antitumor responses in clinical trials, with less toxicity than has been seen with prior immune therapies such as interleukin 2 and ipilimumab. Pembrolizumab, an anti–PD-1 antibody, recently gained US Food and Drug Administration (FDA) accelerated approval for the treatment of patients with ipilimumab-refractory melanoma, while nivolumab, another anti–PD-1 antibody, and MPDL3280A, an anti–programmed cell death 1 ligand (PD-L1) antibody, have been granted FDA “breakthrough designation” for treatment of subsets of patients with refractory Hodgkin lymphoma and metastatic bladder cancer, respectively. Encouraging antitumor activity has also been seen with these agents in patients with other malignancies, including non–small-cell lung cancer and head and neck cancer, tumors not previously thought to be immune-responsive. PD-L1 expression has emerged as a potential predictive biomarker for PD-1–directed therapy. Multiple, distinct, companion assays for PD-L1 positivity have been developed, but there is as yet no comparison, standardization, or prospective validation of these assays. PD-L1 expression on tumor cells and/or the tumor-immune infiltrate is likely only part of the predictive model necessary for selecting patients predisposed to respond to monotherapy. Additional predictive biomarkers are necessary to identify patients most likely to benefit from PD-1–based combination therapy, since tumor cell PD-L1 expression appears to have limited predictive value in this setting.
Introduction
Historically, active immune therapies such as interleukin 2 (IL-2) produced durable responses in only a small minority of patients while being associated with significant, nearly universal toxicity. This has limited their applicability to selected patients, usually with either melanoma or renal cell cancer (RCC) treated at experienced centers. The immune checkpoint inhibitor ipilimumab, while being better tolerated than high-dose (HD) IL-2, also exposes patients to a risk of significant toxicity. Therefore, there has been considerable effort over the past decade to identify patients most likely to benefit from these agents in order to enhance their therapeutic index. The discovery and availability of alternative treatment options for patients with advanced melanoma (BRAF inhibitor therapy) and RCC (vascular endothelial growth factor [VEGF]-targeted therapy) in the last decade have intensified the need to identify those patients who will not benefit from immunotherapy, so as not to delay the use of these more generally accessible alternative therapies, particularly in symptomatic patients. Programmed cell death 1 (PD-1) pathway–blocking antibodies appear to exhibit an improved therapeutic index, relative to HD IL-2 and ipilimumab, and efficacy against an expanded array of tumor types (eg, non–small-cell lung cancer (NSCLC), bladder cancer, head and neck cancer), each of which has a distinct array of standard treatments. This has further highlighted the need for clinically useful biomarkers that can help determine how best to incorporate these new agents into treatment algorithms for patients with specific diseases. Finally, identifying factors that predict the subpopulations of patients and tumor types that respond to immunotherapy is critical to both understanding the mechanism of action of immunotherapy and the efficient development of novel immunotherapy combinations.
Definition of Biomarkers
A biomarker is a biologic molecule, such as a protein or gene, that is measureable in tissue, blood, or other body fluids, and is an indicator of some clinically significant condition. Biomarkers can be diagnostic, surrogate, prognostic, or predictive. While the gold standard of diagnosis in oncology is a pathologic tissue review, a highly elevated prostate-specific antigen (PSA) level in the right clinical setting can be diagnostic of prostate cancer. Although the value of PSA level as a diagnostic biomarker is limited by its sensitivity and specificity, it can be an excellent surrogate biomarker for monitoring prostate cancer response to treatment. Prognostic biomarkers refer to markers that correlate with the natural progression or aggressiveness of a disease. Prognostic biomarkers are useful for informing patients about the risk of recurrence or median survival for their particular type of malignancy and for minimizing confounding factors when analyzing clinical trial cohorts or when prospectively stratifying patients in randomized clinical trials. Predictive biomarkers are defined by their role in predicting a response to a given treatment. Therefore, these are most useful if they can be assessed before the initiation of treatment. This review will focus on predictive biomarkers, as these are most relevant to decision making regarding immunotherapy selection; we will only mention prognostic or surrogate markers to the extent that such discussion sheds potential light on the mechanisms underlying the value of a predictive biomarker.
Biology of the Immune Response
The immune system is controlled by a delicate balance of immune-stimulatory and immune-inhibitory forces that enable the rapid destruction of cells expressing foreign antigens while preventing uncontrolled destruction of normal tissues. The inhibitory forces include regulatory T cells (Tregs), immunosuppressive cytokines, and immune checkpoints, such as the cytotoxic T-lymphocyte antigen 4 (CTLA-4)/B7 and the PD-1/programmed cell death 1 ligand (PD-L1) pathways. For example, IL-2 not only activates CD8+ effector T cells and subsets of natural killer cells, but also CD4+CD25+ and CD4+Foxp3+ Tregs,[1] which constitutively express the IL-2 receptor alpha chain. Studies in viral models of acute and chronic infections shed light on this phenomenon. PD-1 expression is induced by activation of T lymphocytes, and after a successful immune response has eliminated the foreign antigen, PD-1 expression decreases.[2,3] If the immune response is unsuccessful in eliminating the antigen-expressing cells, as in cancer or chronic infections, prolonged antigen stimulation leads to elevated PD-1 expression and induction of the expression of immune-inhibitory ligands, such as PD-L1 or PD-L2, on tumor cells and infiltrating immune cells. The binding of PD-1–expressing T cells to PD-L1 or PD-L2 leads to T-cell dysfunction and “exhaustion,” and results in eventual escape from immune elimination. In addition to this model of adaptive immune inhibition or resistance, as illustrated in melanoma,[4] other tumors may evade immune surveillance by overexpression of PD-L1 secondary to genetic amplification[5] or as a result of aberrant signaling.[6]
Some tumors have an inflamed phenotype, while others have only a sparse immune infiltrate. While the extent of the immune infiltrate can be a good prognostic indicator in some cancers,[7,8] the antitumor response is clearly insufficient to prevent disease progression. In inflamed tumors, negative immune-regulatory factors tend to dominate due to the chronic nature of the immune infiltrate, as illustrated in Gajewski et al (Figure 1A).[9] Therefore, it is hypothesized that patients with tumors containing T-cell infiltrates might be induced to respond to immunotherapy if the immune cells within the tumor microenviroment can be reactivated.[10]
Predictive Biomarkers for Response to Cytokine Therapy-a Proof of Principle
IL-2 received US Food and Drug Administration (FDA) approval for the treatment of patients with RCC or melanoma in the 1990s based on its ability to produce durable antitumor responses in a small minority of patients, an effect that was largely restricted to the high-dose regimen (HD IL-2). While tumor responses were seen in only 15% to 25% of patients, the 6% to 10% of patients who exhibited tumor responses lasting more than 30 months were likely cured. In retrospective analyses of patients with advanced melanoma, IL-2 response has been associated with normal serum lactate dehydrogenase levels, or low plasma VEGF and fibronectin levels,[11] as well as with tumors containing mutations in BRAF or NRAS. Prospective validation of these largely clinical features of response is ongoing. In patients with RCC, tumor response to IL-2 was largely restricted to patients with clear-cell tumors.[12,13] Further, while initial studies suggested that high levels of carbonic anhydrase 9 (CA9) expression by RCC cells predicted for response to IL-2, a prospective clinical trial failed to confirm this finding, supporting a modicum of caution regarding the value of inherent tumor features in predicting response to immunotherapy.
One of the more impressive surrogate markers for the benefit of cytokine therapy is induced or increased autoimmunity. IL-2 responses and the benefit of adjuvant interferon (IFN) appeared more common in patients who exhibited autoimmune phenomena such as thyroiditis or vitiligo. These entities frequently were accompanied by enhanced titers of autoantibodies, suggesting that responses were both autoimmune in nature and more common in patients who were predisposed to develop autoimmunity.[14,15] More recent studies have suggested that response to IL-2 is associated with enhancement of a preexisting gene expression pattern within the tumor that is associated with immune-mediated, tissue-specific destruction under the control of the T helper 1 (Th1)-type cytokine, IFN-γ.[16] Benefit from vaccination has also been linked to tumors expressing an IFN-driven chemokine signature.[9,17] In addition, preliminary results suggest that IL-2 response in patients with RCC may be correlated with tumor cell surface expression of PD-L1,[18] which, as mentioned previously, is frequently associated spatially with tumor-reactive CD8+ T cells in the microenvironment.[4] Taken together, these data suggest that effective immunotherapy may require the preexistence of tumor-specific immunity within the microenvironment and the use of agents, such as HD IL-2 or IFN, in a subset of patients in whom these agents drive effector T-cell function more potently than immune-regulatory signals. While prospective validation of this hypothesis is necessary, it suggests that approaches that could block immunoregulatory signals might also alter the balance within the tumor microenvironment in such predisposed patients, leading to a shift from immune tolerance of a tumor to an active antitumor immune response. This concept has foreshadowed the effectiveness of checkpoint blockade and informed the predictive biomarker work with such agents.
Clinical Results of Checkpoint Blockade-Efficacy and Toxicity
CTLA-4 was the first of the immune checkpoints to be therapeutically targeted in cancer. Ipilimumab, a fully human immunoglobulin (Ig) G-1 monoclonal antibody blocking CTLA-4, improved overall survival in patients with metastatic melanoma and received FDA approval in 2011.[19,20] As with HD IL-2, the great majority of responders alive at 2 years were still alive long after therapy was completed. Although acute side effects from ipilimumab administration were minimal, patients did sometimes develop immune-related adverse events weeks into therapy that required aggressive immunosuppression in order to control.[21] Similar to the autoimmunity described with IL-2 therapy, baseline presence and induction of NY-ESO-1 antibodies have been associated with clinical benefit from ipilimumab.[22] Specific ipilimumab-associated, immune-mediated adverse effects, such as hypophysitis, have been associated with the development of autoantibodies targeting the affected organ.[23]
PD-1 and its ligands, PD-L1 and PD-L2, are also members of the B7-CD28 superfamily and form an immune-checkpoint pathway. Blocking the binding site of the PD-1 receptor and the ligand PD-L1 with monoclonal antibodies also has shown impressive antitumor effects in patients in multiple tumor types,[24-26] including those not previously thought to be immune-responsive. The PD-1/PD-L1 pathway appears to induce peripheral tolerance of the adaptive immune system.[27] Unlike in CTLA-4 knockout mice, which exhibit lethal autoimmunity within weeks of birth,[28] mice in which PD-1 or PD-L1 is knocked out require some additional initiator to develop an autoimmune phenotype.[27,29-31] Clinically, blockade of the PD-1/PD-L1 pathway appears to produce fewer immune-related adverse events than ipilimumab. This improved side-effect profile and broad span of antitumor efficacy have resulted in extremely active drug development in recent years. Pembrolizumab is the first of this anti–PD-1 pathway family of checkpoint inhibitors to gain FDA–
accelerated approval for the treatment of ipilimumab-refractory melanoma. The phase III clinical trial comparing dacarbazine with nivolumab, an anti–PD-1 monoclonal antibody, recently was stopped early due to improved overall survival for the patients who received anti–PD-1 therapy, and nivolumab has already been approved in Japan. In the United States, nivolumab has received breakthrough status for Hodgkin lymphoma. MPDL3280A, an anti–PD-L1 monoclonal antibody, has received breakthrough status for the treatment of bladder cancer. Development of PD-1/PD-L1 pathway–blocking agents in many other tumors is underway, given the responses seen in early trials.
Initial single-arm trials of combination checkpoint blockade (ipilimumab and nivolumab) have shown potential to significantly improve response rates.[32,33] However, they also appear to increase the number of grade 3 and 4 side effects. The ipilimumab + nivolumab combination is currently being further investigated, including in phase III trials in which the efficacy and toxicity of the combination can be compared with either nivolumab monotherapy or standard of care. While the increased antitumor efficacy of combination immune-modulatory therapies is encouraging, such combinations may not only significantly increase the toxicity but also the cost of therapy. Thus, the development of predictive biomarkers for the routing of patients to the most appropriate therapies is essential to maximize clinical benefit and minimize toxicity. Predictive biomarkers may also facilitate use of checkpoint inhibitors in patients with high-risk resected disease, since a percentage of patients treated in the adjuvant setting may already be cured, and thus the toxicity and treatment expense may be harder to justify.
Biomarkers and Immune Checkpoints
Clearer insight into the biology of a therapeutic target facilitates its clinical development. Therefore, it may prove easier to define predictive biomarkers for individual immune-checkpoint inhibitors than was the case for pleiotropic cytokines such as IL-2 or IFNs. Despite the role of autoimmunity as a surrogate for response to cytokine therapy, its role in CTLA-4 and PD-1 immune-checkpoint pathway–blocking therapy is less clear. Both CTLA-4 and PD-1/PD-L1 play important roles in regulating T-lymphocyte tolerance, and both have been implicated in human autoimmune disease.[34] In addition, both ipilimumab and, to a lesser extent, PD-1–pathway inhibitors induce immune-related toxicities that appear to be autoimmune in nature. The relationship of these toxicities to tumor response with these agents is unclear, and because trials to date have generally excluded patients with a history of autoimmune disease, the extent to which a predisposition to autoimmunity predicts for antitumor activity remains to be established. Studies are actively investigating not only predictive biomarkers for efficacy but also for toxicity. Moving forward, a patient’s germline genetics also may play a critical role in predicting the clinical effects of various immune therapies, as has already been proposed in the case of ipilimumab.[35,36]
While pretreatment gene expression profiling of tumors has suggested that those with high baseline expression levels of immune-related genes are more likely to respond to ipilimumab,[37] few biomarkers in ipilimumab studies have been truly predictive in nature. For example, the appearance of ICOS+CD4+ T lymphocytes after ipilimumab treatment has been proposed as a pharmacodynamic biomarker that can be used to assess adequate biologic response to treatment.[38] While early reports suggested that an absolute lymphocyte count > 1,000/μL after two doses of ipilimumab[39] correlated with clinical benefit and overall survival,[39] Postow et al reported that nearly all patients who received ipilimumab had a significant increase in their absolute lymphocyte count, potentially mitigating the value of this observation.[40] In addition, investigators have reported that absence of tumor-infiltrating lymphocytes at 4 weeks into therapy or an increase in myeloid-derived suppressor cell (MDSC) numbers in the peripheral blood over the first 24 weeks were both negatively associated with clinical benefit from ipilimumab therapy.[41] In any event, since these studies measured pharmacodynamic markers of treatment effect, they do not inform the choice of patients for ipilimumab therapy.[38] Of note, in a small cohort of 26 patients receiving ipilimumab at 10 mg/kg, a lower number of MDSCs in the baseline peripheral blood was predictive of overall survival.[41] However, much like the negative association of pretreatment serum VEGF level with clinical benefit and overall survival after ipilimumab,[42] baseline MDSC count and serum VEGF level may be functioning as prognostic rather than predictive markers.[37]
Some uncertainty about the mechanism of action of ipilimumab in the tumor microenvironment may be confounding the ability to develop predictive biomarkers of benefit. Given that tumor-infiltrating lymphocytes likely express many immune checkpoints and are already deactivated, it has been postulated that the mechanism of action of ipilimumab in tumors is to target Tregs, as suggested by mouse studies.[43] Thus, ipilimumab’s primary antitumor effect may not be unblocking T-cell activation at initial tumor-antigen presentation but targeting Tregs for destruction within the tumor microenvironment. In support of this concept, Hamid et al reported that elevated Foxp3 and IDO expression in baseline tumor biopsies was associated with clinical benefit from ipilimumab.[44] This and other reports suggest that, as with IL-2, tumors with an inflamed microenvironment may respond better to ipilimumab, since the presence of immunosuppressive factors such as Tregs may not be as much of an obstacle to its efficacy.[10]
Biomarkers for the PD-1/PD-L1–Pathway Inhibitors
PD-L1 expression in tumors is one of a number of different immunosuppressive factors proposed in the Gajewski model (Figure 1) of chronically inflamed tumors with an immunosuppressed microenvironment. In preclinical models, expression of PD-L1 allows tumors to evade tumor-specific effector T-cell cytotoxicity.[45] Clinically, PD-L1 is expressed by many tumor types and is associated with worse prognosis in several, including lung adenocarcinoma and RCC.[46,47] PD-L1 expression is IFN-γ–inducible and can be on either the immune infiltrate or the tumor cells (Figure 2). Patients whose kidney tumors had ≥ 10% tumor cell expression with the murine anti–human PD-L1 antibody (clone 5H1) had a threefold increased risk of dying of their disease. By contrast, PD-L1 expression on some tumors, most notably melanoma, appears to be associated with a good prognosis.[48] The mechanisms underlying this prognostic diversity and the relative importance of PD-L1 expression in various cell populations within the tumor have yet to be fully elucidated. Nonetheless, the critical role played by PD-L1 expression in tumor immunobiology has clearly identified this pathway as an important target for checkpoint-inhibitor therapy.
Early clinical trials with PD-1 pathway–blocking agents have produced exciting clinical responses in multiple tumor types, including some previously believed to be unresponsive to systemic immunotherapy (Figure 3).[49] Tumor PD-L1 expression was initially explored as a potential predictive biomarker in the single-dose pilot phase I study of nivolumab.[24] This exploratory analysis consisted of tumor specimens from nine patients. PD-L1 expression on tumors by immunohistochemical analysis was performed with clone 5H1 mentioned above.[50] While three of four patients with PD-L1 expression on the tumor cell surface responded to nivolumab (75% response rate), all of the five tumors that did not express any membranous PD-L1 did not respond to treatment. In a follow-up study performed in conjunction with the multidose, phase I nivolumab trial, 9 of 25 patients with PD-L1–expressing tumors responded to treatment, while none of 18 patients with PD-L1–nonexpressing tumors responded (Figure 3). These preliminary data suggest that tumor cell expression of PD-L1, using the clone 5H1 assay, could be a strong predictor of response to nivolumab treatment.
Unfortunately, several subsequent observations have suggested that the predictive value of PD-L1 expression may not be as clear-cut as initially reported. As previously mentioned, PD-L1 could also be expressed on infiltrating lymphocytes, monocytes, and macrophages (Figure 2).[47] Taube et al explored the predictive function of the expression of PD-L1 and PD-L2 on tumor cells and of PD-1 on the immune infiltrate.[51] They found that the expression of PD-L1 on tumor cells and immune cells was highly associated with PD-1 expression on infiltrating lymphocytes-yet PD-L1 expression on tumor cells had the strongest association with response to nivolumab. Based on these data, Bristol-Meyers Squibb developed an automated assay (using the 28-8 clone) that defined > 5% tumor cell membranous expression of PD-L1 to be positive.[47,52,53] However, the other two companies (Genentech/Roche and Merck) leading in the development of PD-1–pathway blockers have developed distinct companion assays for PD-L1 expression, each with its own anti–PD-L1 antibody. The Genentech/Roche assay measures PD-L1 expression on immune-infiltrating cells, which investigators claim best enriches for responders to MPDL3280A.[54-57] This assay predicted for benefit from MPDL3280A in patients with bladder cancer, contributing to this antibody receiving breakthrough status for study in patients with PD-L1–positive metastatic bladder cancers. The Merck PD-L1 assay, developed in conjunction with pembrolizumab, defined PD-L1 positivity in melanoma specimens as tumor surface expression (> 1%).[58,59] However, variations in this assay and its application have emerged across tumor types. For example, in NSCLC patients, both immune and tumor cells were included in the cut-off (Figure 3, Gandh et al),[60] while in the head and neck cancer study, only patients with some PD-L1 expression on tumor cells were enrolled (Figure 3, Seiwert et al).[61] Since these assays have not been compared, it is impossible to ascertain whether these differences in definitions are a function of the assay (antibody specificity), of the biologic differences between anti–PD-1 and anti–PD-L1 therapy, or of the nature of the patient samples analyzed. Thus, it is hard to determine whether there is any consistency in the tumors that are declared to be PD-L1–positive. Clearly, some sort of standardized definition of PD-L1 positivity that links these various assays is necessary to facilitate the study of PD-L1 as a predictive biomarker for PD-1–pathway blockade. Lacking this, comparing clinical results across studies in biomarker-defined subsets of patients will be especially hazardous.
Further complicating matters, analyses with these different companion assays (such as an automated PD-L1 antibody assay used in conjunction with nivolumab) have suggested that, in contrast to earlier results, many patients with PD-L1 negative tumors can respond to PD-1–pathway blockade, including some who have exhibited a complete response (Figure 3).[49] Nonetheless, despite the significant number of false negatives, it is notable that across multiple tumor types, with four different therapies and four different assays for PD-L1 expression, there was a clear trend for PD-L1–positive tumors to respond better than PD-L1–negative tumors. In addition, the degree of PD-L1 expression, by whatever assay, may be positively correlated with response. Therefore, establishing a more stringent cut-off for PD-L1 expression would better enrich for responding patients but fail to identify a larger absolute number of potential responders, while a less stringent PD-L1 expression cut-off might capture more of the responding patients but reduce the predictive power of the assay. An example of this dynamic in patients with melanoma is shown in the Table.[52] Also, tumors are heterogeneous, and PD-L1 expression is inducible on both immune cells and tumor cells. Therefore, the sample used for the assay may not be representative of the whole tumor. Further, PD-L1 expression may be discordant between the primary tumor and the metastatic lesions that are being targeted by the immune therapy. For example, preliminary data suggest moderate discordance in RCC PD-L1 expression between primary and metastatic lesions. Whether this discordance has been a result of tissue preparation and storage issues or biologic differences is uncertain. However, it is perhaps notable that higher PD-L1 expression was associated with higher Fuhrman grade, a factor that was more variable in the primary than the metastatic lesions. Not surprisingly, therefore, the metastatic lesions were typically more homogeneous for PD-L1 expression than the primary tumors.[62] Finally, tumor PD-L1 expression may be less relevant as a predictive biomarker for combination immunotherapy. For example, the combination of ipilimumab and nivolumab actually produced similar response rates for PD-L1–positive and –negative tumors.[32,33] Further, tumor objective response rates for combinations of PD-1–blocking agents with non–immune-based therapies may also be less likely to be associated with PD-L1 expression.[63] For all of these reasons, PD-L1 expression is not an optimal biomarker for patient selection, and lack of PD-L1 expression cannot be reliably used to exclude patients from treatment with PD-1–pathway blockade.
Nonetheless, PD-L1 expression can be a useful predictive biomarker in several capacities. The data in Table 1 clearly suggest that PD-L1 expression, by whatever assay, can enrich for patients who can respond to single-agent PD-1–pathway blockade. Thus, PD-L1 expression could serve as a stratification factor in randomized trials comparing different types of therapies. Further, data suggest that tumor types lacking a consistent percentage of PD-L1 expression are less likely to benefit from single-agent PD-1–pathway blockade.[45]
Therefore, the extent of PD-L1 expression in a tumor type might encourage the inclusion of such tumors in clinical trials. Such an approach has predicted the responsiveness of head and neck cancer and bladder cancer,[56,61] and has fostered investigation of PD-1–pathway blockade in other highly PD-L1–positive tumors, such as Merkel cell carcinoma and Hodgkin lymphoma.[64,65]
Selecting a stringent cut-off for PD-L1 expression might identify a subset of patients with sufficiently high benefit from PD-1–pathway blockade to justify the exploration of single-agent treatment either compared with standard therapy in randomized trials or in single-arm trials in a setting where there is no curative treatment approach. Such strategies have been employed for the development of PD-1–pathway inhibitors in bladder cancer, head and neck cancer, and NSCLC.
Finally, a low level of PD-L1 expression might identify a group of patients who would be best treated with combination immunotherapy. While a prospective trial will be necessary to validate a role for PD-L1 as a predictive biomarker, the data in patients with metastatic melanoma suggest that this may be a reasonable criterion for selecting patients who should be treated with the ipilimumab/nivolumab regimen. Optimal agents to combine with a PD-1–pathway blocker might be those capable of inducing immune infiltration and PD-L1 expression within the tumor. However, given the heterogeneity of many tumors, particularly primary specimens, and technical issues related to tissue processing and storage, if PD-L1 expression is to be used for any of these purposes, it would seem prudent to analyze pretreatment biopsy specimens from metastatic lesions whenever possible.
In addition, understanding the biology of PD-L1 expression in tumors may contribute to the development of better predictive markers and models for assessing response to checkpoint inhibitor therapy. This may be particularly useful in determining why some patients with PD-L1–positive tumors fail to respond to therapy. In some cases this could be because the PD-L1 expression is constitutive, driven by activation of a pathway within the tumor (eg, PI3-kinase), rather than induced by an adaptive response to tumor-specific CD8+ lymphocyte infiltration. Indeed, if PD-L1 is overexpressed on a tumor lacking an appropriate immune infiltrate, blockade may have no effect because the tumor is lacking the effector cells that fight the cancer. Therefore, some measure of immune infiltration (whether assessment of immune phenotypes, as suggested by the Immunoscore research, or gene expression profiles linked to chemokine expression) together with PD-L1 expression might be a better predictor of tumor response.[7,66] Such a model for a dual biomarker has been proposed by Sznol et al (Figure 4).[67]
Alternatively, resistance to treatment in this population might suggest that other immune-suppressive factors may be downregulating the immune response. Understanding the factors involved in a particular tumor type might be the key to the development of novel PD-1/PD-L1–based combination therapies, such as those targeting additional immune checkpoints (eg, TIM-3 [T-cell immunoglobuilin and mucin domain–containing protein 3] or LAG-3 [lymphocyte activation gene 3 protein]) or other immunosuppressive factors (eg, Tregs, IDO, or MDSCs).
Assessing Potentially Immune-Responsive Tumors by Genomics
Mutational burden varies greatly between tumors. Many tumors that respond to PD-1–pathway inhibitors, such as melanoma, NSCLC, and bladder cancer, are those with a high mutation load.[68] While the mutational load in melanoma was not sufficient to predict response to ipilimumab, it was significantly associated with clinical benefit (P = .028, complete response vs no response, Fisher’s exact test).[69] It is interesting that PD-1–pathway inhibitors also have shown responses in tumors-such as bladder cancer, NSCLC, and head and neck cancer-that are associated with smoking, a known exogenous mutagen. Assaying the neoantigens created within these tumors that might be presented by the patient’s major histocompatibility complex may better identify tumors able to induce immune responses that can be restored by immune-checkpoint inhibition.[70] Further, it is conceivable that the presence of such recognizable neoantigens may also one day form a component of a predictive biomarker model for response to checkpoint blockade.
Conclusion
Immune-checkpoint inhibition clearly has a significant role in the future of oncology. Multiple agents in this class have shown remarkable antitumor effects in a wide variety of clinical trials. While intratumoral PD-L1 expression appears to predict better responses to monotherapy with PD-1 pathway–blocking agents, it is unclear whether this will translate into a clinically useful predictive biomarker in isolation. Characterizing tumors by PD-L1 expression, immune infiltration, chemokine signature, and tumor mutational frequency may be a means of creating an integrated model for determining which patients may benefit from which immune-checkpoint inhibitors, either alone or in combination. Further, the identification of tumors lacking such biomarkers might prove useful in selecting patients for alternative therapies, including adoptive transfer of genetically modified T cells. Taken together, the identification and application of such markers are critical to the rational development and application of immune therapies.
Financial Disclosure: Dr. Atkins serves as a consultant to Amgen, Bristol-Myers Squibb, Genentech/Roche, Merck, and NeoSTem. Dr. Mahoney has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. van der Vliet HJ, Koon HB, Yue SC, et al. Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res. 2007;13:2100-8.
2. Vibhakar R, Juan G, Traganos F, et al. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp Cell Res. 1997;232:25-8.
3. Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998; 188:2205-13.
4. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127-37.
5. Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268-77.
6. Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007; 13:84-8.
7. Ascierto PA, Capone M, Urba WJ, et al. The additional facet of Immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54.
8. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199-209.
9. Gajewski TF, Fuertes M, Spaapen R, et al. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286-92.
10. Gajewski TF, Chesney J, Curriel TJ. Emerging strategies in regulatory T-cell immunotherapies. Clin Adv Hematol Oncol. 2009;7:1-10.
11. Sabatino M, Kim-Schulze S, Panelli MC, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27:2645-52.
12. Cangiano T, Liao J, Naitoh J, et al. Sarcomatoid renal cell carcinoma: biologic behavior, prognosis, and response to combined surgical resection and immunotherapy. J Clin Oncol. 1999;17:523-8.
13. Upton MP, Parker RA, Youmans A, et al. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28:488-95.
14. Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354: 709-18.
15. Koon H, Atkins M. Autoimmunity and immunotherapy for cancer. N Engl J Med. 2006;354:758-60.
16. Weiss GR, Grosh WW, Chianese-Bullock KA, et al. Molecular insights on the peripheral and intratumoral effects of systemic high-dose rIL-2 (aldesleukin) administration for the treatment of metastatic melanoma. Clin Cancer Res. 2011;17:7440-50.
17. Ulloa-Montoya F, Louahed J, Dizier B, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31:2388-95.
18. Bailey AS, Cheng S, Kwon ED, et al. PDL-1/PDL-3 (programmed death ligand-1/3) tissue expression and response to treatment with IL2 and antiangiogenic therapies. J Clin Oncol 2013;31(suppl):abstr 4521.
19. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
20. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-26.
21. Weber JS. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am Soc Clin Oncol Educ Book. 2012;174-7.
22. Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108:16723-8.
23. Iwama S, De Remigis A, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6:230ra45.
24. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-75.
25. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-65.
26. Segal NH, Antonia SJ, Brahmer JR, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol. 2014;32(suppl 5S):abstr 3002.
27. Wang J, Yoshida T, Nakaki F, et al. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102: 11823-8.
28. Khattri R, Auger JA, Griffin MD, et al. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162:5784-91.
29. Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477-83.
30. Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141-51.
31. Kasagi S, Kawano S, Okazaki T, et al. Anti-programmed cell death 1 antibody reduces CD4+PD-1+ T cells and relieves the lupus-like nephritis of NZB/W F1 mice. J Immunol. 2010;184:2337-47.
32. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-33.
33. Hammers H, Plimack E, Infante J, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2014; 32(suppl 5S):abstr 4504.
34. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239-45.
35. Breunis WB, Tarazona-Santos E, Chen R, et al. Influence of cytotoxic T lymphocyte-associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J Immunother. 2008;31:586-90.
36. Adaniel C, Rendleman J, Polsky D. Germline genetic determinants of immunotherapy response in metastatic melanoma. J Clin Oncol 2014;32(suppl 5S):abstr 3004.
37. Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019-31.
38. Ng Tang D, Shen Y, Sun J, et al. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1:229-34.
39. Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767-75.
40. Postow MA, Chasalow SD, Yuan J, et al. Pharmacodynamic effect of ipilimumab on absolute lymphocyte count (ALC) and association with overall survival in patients with advanced melanoma. J Clin Oncol. 2013;31(suppl):abstr 9052.
41. Kitano S, Postow MA, Cortez C, et al. Myeloid-derived suppressor cell quantity prior to treatment with ipilimumab at 10mg/kg to predict for overall survival in patients with metastatic melanoma. J Clin Oncol.2012;30(suppl):abstr 2518.
42. Yuan J, Zhou J, Dong Z, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2014;2:127-32.
43. Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32-42.
44. Hamid O, Chasalow, SD, Tsuchihashi Z, et al. Association of baseline and on-study tumor biopsy markers with clinical activity in patients (pts) with advanced melanoma treated with ipilimumab. J Clin Oncol. 2009;27:15(suppl):abstr 9008.
45. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-54.
46. Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682-8.
47. Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004; 101:17174-9.
48. Gadiot J, Hooijkaas AI, Kaiser AD, et al. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117:2192-201.
49. Callahan MK. Understanding the biology behind responses to immunotherapy. Presented at ASCO Annual Meeting; 2014 May 30–June 4; Chicago, IL. Available from: hjttp://meetinglibrary.asco.org/content/94863?media=vm. Accessed October 8, 2014.
50. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381-5.
51. Taube JM, Klein AP, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5065-74.
52. Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol. 2013;31(suppl):abstr 3016.
53. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311-8.
54. Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31(suppl):abstr 9010.
55. Herbst RG, Gordon MS, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol. 2013;31(suppl):abstr 3000.
56. Powles T, Vogelzang NJ, Fine GD, et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC). J Clin Oncol. 2014;32(suppl 5S):abstr 5011.
57. Soria JC, Cruz C, Bahleda R, et al. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). Eur Cancer Congr 2013 (ECCO-ESMO-ESTRO); September 27–October 1, 2013; Amsterdam, The Netherlands. Abstr 3408.
58. Daud AI, Hamid O, Ribas A, et al. Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma (MEL): correlation of tumor PD-L1 expression with outcome. American Association for Cancer Research Annual Meeting 2014; April 5–9, 2014; San Diego. Abstr CT104.
59. Ribas A, Hodi FS, Kefford, R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). J Clin Oncol 2014;32(suppl 5S):abstr LBA9000.
60. Gandhi LB, Balmanoukian A, Hui, R, et al. MK-3475 (anti-PD-1 monoclonal antibody) for non-small cell lung cancer (NSCLC): antitumor activity and association with tumor PD-L1 expression. American Association for Cancer Research Annual Meeting 2014; April 5–9, 2014; San Diego. Abstr CT105.
61. Seiwert TB, Burtness B, Weiss J, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV–associated head and neck (H/N) cancer. J Clin Oncol. 2014;32(suppl 5S): abstr 6011.
62. Callea M, Genega EM, Gupta M, et al. PD-L1 expression in primary clear cell renal cell carcinomas (ccRCCs) and their metastases. J Clin Oncol. 2014; 32(suppl 4):abstr 467.
63. Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2014;32(suppl 5S):abstr 5010.
64. Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462-73.
65. Afanasiev OK, Yelistratova L, Miller N, et al. Merkel polyomavirus-specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19:5351-60.
66. Messina JL, Fenstermacher DA, Eschrich S, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012;2:765.
67. Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021-34.
68. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214-8.
69. Snyder Charen A, Makarov V, Merghoub T, et al. The neoantigen landscape underlying clinical response to ipilimumab. J Clin Oncol. 2014;32(suppl 5S): abstr 3003.
70. Fritsch EF, Rajasagi M, Ott PA, et al. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol Res. 2014;2:522-9.
71. Grosso JF, Horak CE, Cardona DM, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558;ONO-4538). Presented at the American Society of Clinical Oncology 2013 Annual Meeting; May 31–June 4, 2013; Chicago, IL. Poster 3016.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.