R115777 May Be Effective Against Certain Subtypes of Recurrent Glioblastoma Multiforme
LOS ANGELES-The North American Brain Tumor Consortium (NABTC) reported that R115777 (Zarnesta) shows modest activity against recurrent glioblastoma multiforme and might be effective as a therapy for certain subtypes of these
LOS ANGELESThe North American Brain Tumor Consortium (NABTC) reported that R115777 (Zarnesta) shows modest activity against recurrent glioblastoma multiforme and might be effective as a therapy for certain subtypes of these aggressive brain tumors (ASCO abstract 317).
The phase II study involved 42 glioma patients, 33 with glioblastoma multiforme. Five of those 33 responded to R115777, according to lead investigator Timothy Cloughesy, MD, director of the Neuro-Oncology Program at the University of California, Los Angeles. Three had partial responses, and two had stable disease. Four are without progression for greater than 15 months, he said, describing the response as durable. The fifth died of pneumonia believed to be unrelated to the treatment. Median time to progression with R115777 was no better than current therapies, 8 weeks.
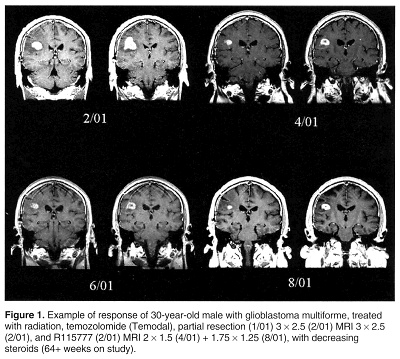
"There seems to be a subpopulation that responds," Dr. Cloughesy said, describing the results as encouraging but not specific enough to predict who would benefit. "One of the goals is to define the subpopulation that responds," he said. "Investigators are attempting to identify this group. There are many leads but no clear answer yet."
Dr. Cloughesy and his colleagues concluded that R115777 is tolerable in the patient population that has been tested, when delivered orally at 300 mg bid in repeating 28-day cycles of 21 days on-drug and 7 days off-drug. Toxicity forced three patients to go off trial. Grade 3/4 adverse events included three cases of granulocytopenia and one each of depressed consciousness, constipation, leukopenia, photophobia, skin rash, and thrombocytopenia.
Second Phase II Trial
An experimental molecular therapy, R115777 inhibits farnesyl transferase, an enzyme that activates a number of proteins, including the Ras oncogene. R115777 has shown activity against leukemia and breast cancer, and the NABTC plans to study R115777 in combination with external beam radiation for glioblastoma multiforme.
But first, a second phase II trial of R115777 in recurrent glioblastoma multiforme is planned, with patients taking enzyme-inducing anti-epileptic drugs (EIAEDs) for protection against seizures. None of the patients in the previous phase II trial were taking EIAEDs, although the drugs are commonly given to patients with brain tumors.
EIAEDs sometimes cause the metabolism of the experimental agent to accelerate in patients, Dr. Cloughesy explained. If that happens, finding the right dose of R115777 might be a problem, along with toxicity, he said. Consequently, the consortium plans to enroll a separate cohort of patients on enzyme-inducing agents, and give them the maximum tolerated dose of R115777 to see if and how they respond.