Rapid responses to nilotinib in newly diagnosed early Ph+ CML
ORLANDO-In newly diagnosed, early chronic phase, Ph+ chronic myelogenous leukemia, nilotinib (Tasigna) quickly induced complete cytogenetic responses and was well tolerated, Jorge Cortes, MD, of M.D. Anderson Cancer Center, said at ASH 2007 (abstract 29).
ORLANDO-In newly diagnosed, early chronic phase, Ph+ chronic myelogenous leukemia, nilotinib (Tasigna) quickly induced complete cytogenetic responses and was well tolerated, Jorge Cortes, MD, of M.D. Anderson Cancer Center, said at ASH 2007 (abstract 29).
The trial included 35 newly diagnosed (6 months or less) adult patients with Ph+ CML in early chronic phase who had no prior therapy or less than a month of treatment with interferon-alfa or imatinib (Gleevec). Patients received 400 mg nilotinib twice daily, with dose reductions to 200 mg twice or once daily allowable. Seven patients had received prior imatinib for less than 1 month (patients with minimal prior therapy were eligible). Mean follow-up was 6.5 months.
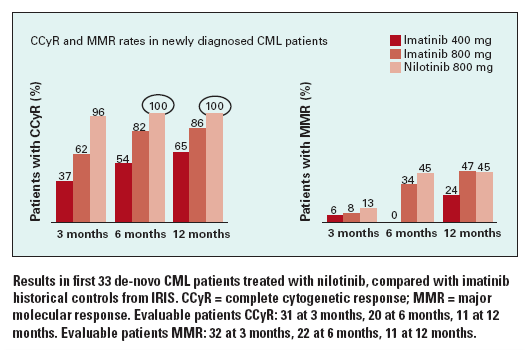
All 33 evaluable patients had a complete hematologic response, and 30 of 31 (96%) with at least 3 months follow-up achieved CCyR. MMRs were reported in 14 of 32 (45%) and complete molecular responses (CMR) in 5 of 32 (16%). Within 3 months of therapy, 97% had a major cytogenetic response (MCR). In addition, MCRs were obtained in all 20 patients with 6 months of follow-up and in all 11 with 12 months.
At 3 months, BCR-ABL transcript levels were reduced by a median of 2-log and at 6 months by 3-log. By 6 months, at least 50% achieved MMR. CCyRs with nilotinib compared favorably with historic imatinib experience (see Figure).