Recap: Use of Tazemetostat in EZH2-Mutant vs Wild-Type Follicular Lymphoma
Expert hematologist-oncologists discuess the E7438-G000-101 clinical trial and consider the EZH2 inhibitor tazemetostat as a treatment for relapsed/refractory follicular lymphoma.

Tazemetostat (Tazverik) is an approved treatment option for patients with follicular lymphoma and an EZH2 mutation who have had 2 prior lines of therapy.1 This therapy offers the opportunity for durable responses in patients with heavily pretreated disease. However, a recent propensity score–matched analysis of the phase 1/2 E7438-G000-101 trial (NCT01897571) found that patients with follicular lymphoma who have EZH2 wild-type disease also may benefit from treatment.2
In a recent edition of Between the Lines hosted by CancerNetwork®, Steven Park, MD, vice chair of research in the Department of Hematologic Oncology and Blood Disorders at Atrium Health’s Levine Cancer Institute in Concord, North Carolina, and Bruce D. Cheson, MD, FACP, FAAAS, FASCO, a hematologic oncologist at the Center for Cancer and Blood Disorders in Bethesda, Maryland, spoke about this study and how patients with wild-type vs EZH2 mutant follicular lymphoma compared in their response to the first-in-class EZH2 inhibitor.
During the discussion, Park and Cheson reviewed the trial, the updated results observed in the propensity score–matched analysis, and additional treatments for patients with follicular lymphoma, specifically ongoing trials involving patients with EZH2 mutations.
Treatment Options for Follicular Lymphoma
Tazemetostat was designed to combat overexpression of EZH2, which can lead to disease progression in follicular lymphoma. When considering treatment, identification of either aggressive or indolent lymphoma is essential as it can help dictate treatment plans. Initial therapy typically consists of treatment with a CD20-based chemoimmunotherapy regimen, with most patients requiring subsequent therapy because of a high rate of relapse.
“I consider tazemetostat as third- or fourth-line therapy, especially in patients who cannot tolerate a more intense regimen. In my experience, tazemetostat is better tolerated compared with conventional chemotherapy with a more favorable adverse effect profile,” Park said.
Cheson highlighted that although there are a number of treatment options for patients with relapsed follicular lymphoma, the number of options has declined recently as the FDA ruled in April that current approvals of notoriously toxic PI3K inhibitors need to be supported by randomized data to support continued use.3 Additional options for treatment include stem cell transplant or CAR T-cell therapy if aggressive follicular lymphoma is identified, and if the disease is indolent, chemoimmunotherapy is typically used in the first-line setting.
Phase 1/2 E7438-G000-101 Trial
Cheson and Park discussed the single-arm trial’s initial results, which were published in Lancet Oncology in 2020.4 The trial analyzed the efficacy and safety of tazemetostat in patients with follicular lymphoma in dose-escalation and -expansion portions. Before matching, a total of 45 patients were enrolled in the EZH2 mutation cohort and 54 in the EZH2 wild-type cohort.
Median follow-up was 22.0 months for the EZH2 mutation cohort vs 35.9 months for the wild-type cohort, with objective response rates (ORRs) of 69% (95% CI, 53%-82%) and 35% (95% CI, 24.9%-40.5%), respectively. Complete responses occurred in 13% of patients in the mutation cohort vs 4% in the wild-type cohort, with partial responses occurring in 56% and 31%, respectively. Of those with responses, 10 patients underwent allogeneic or hematopoietic stem cell transplants, 3 in the mutant and 7 in the wild-type cohort.
In the EZH2-mutated cohort, the median duration of response was 10.9 months (95% CI, 7.2-not estimable [NE]) vs 13.0 months (95% CI, 5.6-NE) in the wild-type cohort. The median progression-free survival (PFS) was 13.8 months (95% CI, 10.7-22.0) in the mutated group vs 11.1 months (95% CI, 3.7-14.6) in the wild-type group.
“What caught my eye was the table comparing the patient characteristics with mutated and wild-type [EZH2]. What hit me was they didn’t match at all. The patients with mutations had 2 prior therapies vs 3 for wild-type disease, with 16% vs 30% having 5 or more [prior therapies],” Cheson said.
Updated Results
In the current analysis, propensity matching produced 28 sets of patients with either EZH2 mutations or wild-type disease. The patient characteristics prior to and after matching can be seen in the Table.2
TABLE. Patient Characteristics of Phase 1/2 E7438-G000-101 Trial vs Propensity Score-Matched Analysis2
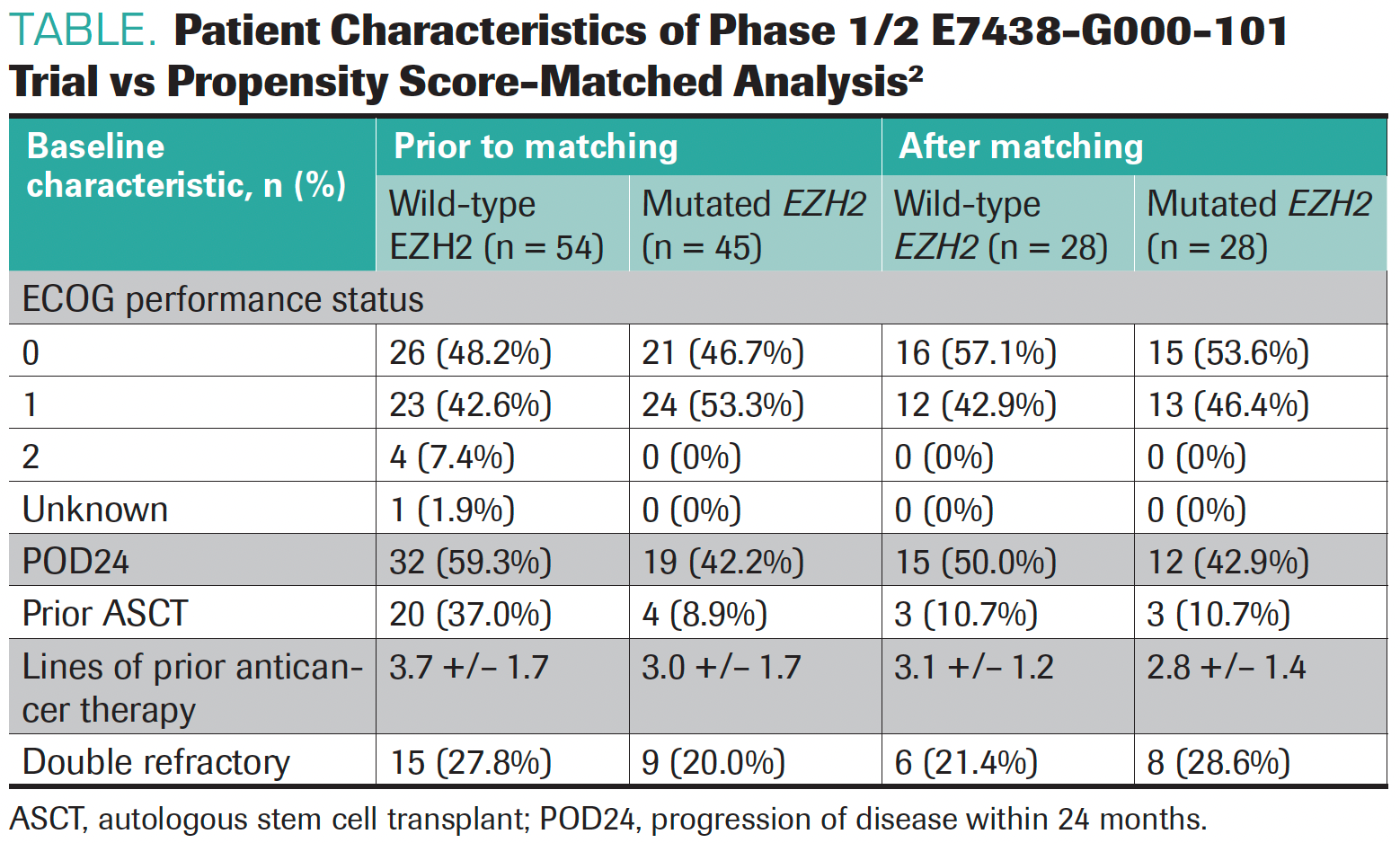
“This is a much better comparison. The first study was not designed to compare the 2 groups head-to-head. In this one, by matching their underlying propensity scores, it is a much better comparison after we normalize different factors and patient characteristics,” Park noted.
The median PFS after matching was 14.8 months in the mutated cohort vs 14.3 months in the wild-type cohort (P = .72). The ORR in the matched study was 71% (95% CI, 54%-88%) for patients in the mutated cohort vs 50% (95% CI, 31%- 69%) in the wild-type cohort.
Both Park and Cheson agreed that this analysis was more fair and comparable. However, the response rates were still higher in those with EZH2 mutations. Park commented that even though there is a higher response rate in one group vs another, the survival outcomes were comparable. Both investigators were intrigued by the outcomes and admitted that further research into tazemetostat in patients with non–EZH2-mutated follicular lymphoma will be important.
Future Treatment of Follicular Lymphoma
Even as treatments advance, there are still unmet needs for patients with follicular lymphoma. Although tazemetostat offers a potential option, those with more aggressive types of lymphoma have limited options. Park noted that CAR T-cell therapy is beginning to look more promising as a treatment for these patients.
Additional treatments for patients with follicular lymphoma involve bispecific antibodies. One such treatment, mosunetuzumab (Lunsumio), was granted priority review in July 2022 for patients with relapsed/refractory follicular lymphoma following at least 2 prior therapies.5 Results were based on the phase 1/2 GO29781 study (NCT02500407), which explored mosunetuzumab as a single agent and in combination with atezolizumab (Tecentriq) in patients with relapsed/ refractory B-cell non-Hodgkin lymphoma or chronic lymphocytic leukemia. A complete response was observed in 60% (95% CI, 49%-70%) of patients with an ORR of 80% (95% CI, 70%-88%). Patients had a median duration of response of 22.8 months (95% CI, 9.7-not evaluable) and a median PFS of 17.9 months.6
“Just based on the data being presented at different meetings, certainly the response rates are high with bispecific antibodies such as mosunetuzumab. Patients who respond have an excellent duration of response,” Park said.
Current combination studies include a phase 1b/3 study (NCT04224493) of tazemetostat with lenalidomide (Revlimid) and rituximab [Rituxan] for patients with relapsed/refractory follicular lymphoma who have 1 or more lines of prior therapy.7 Interim results presented at the 2022 American Society of Clinical Oncology Annual Meeting included data on 43 patients, of whom 34.9% were refractory to rituximab, 25.6% had POD24 of first-line therapy, and 14.6% had an EZH2 mutation.
“This combination appeared very promising with an ORR of 95% and a CR rate of 50%, regardless of their EZH2 mutation status. In this study, [approximately] 80% of the patients were EZH2 wild-type,” Park said.
Moving forward, Park and Cheson agreed that these results and others like them offer optimism for the continued development of treatments for patients with follicular lymphoma.
REFERENCES
- FDA granted accelerated approval to tazemetostat for follicular lymphoma. News release. FDA. June 18, 2020. Accessed September 21, 2022. https://bit.ly/3rfoGI5
- Proudman DG, Gupta D, Nellesen D, et al. Tazemetostat in relapsed/refractory follicular lymphoma: a propensity score-matched analysis of E7438-G000-101 trial outcomes. Oncotarget. 2022;13:677-683. doi:10.18632/oncotarget.28229
- Phosphatidylinositol 3-kinase (PI3K) inhibitors in hematologic malignancies. Oncologic Drugs Advisory Committee meeting. FDA. News release. April 21, 2022. Accessed September 21, 2022. https://bit.ly/36CFphH
- Morschhauser F, Tilly H, Chaidos A, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020;21(11):1433-1442. doi:10.1016/S1470-2045(20)30441-1
- FDA grants priority review to Roche’s Lunsumio for people with relapsed or refractory follicular lymphoma. News release. Roche. July 6, 2022. Accessed September 29, 2022. https://bit.ly/3nGWsUz
- Budde EL, Sehn LH, Matasar M, et al. Mosunetuzumab monotherapy is an effective and well-tolerated treatment option for patients with relapsed/refractory (R/R) follicular lymphoma (FL) who have received ≥2 prior lines of therapy: pivotal results from a phase I/II study. Blood. 2021;138(suppl 1):127. doi:10.1182/blood-2021-145872
- Batlevi CL, Park SI, Phillips TJ, et al. Updated interim analysis of the randomized phase 1b/3 study of tazemetostat in combination with lenalidomide and rituximab in patients with relapsed/refractory follicular lymphoma. J Clin Oncol. 40(suppl 16):7572. doi:10.1200/JCO.2022.40.16_suppl.7572
EP: 1.Relapsed/Refractory Follicular Lymphoma Overview and Treatment Options
EP: 2.Tazemetostat in R/R FL: Original Trial Data and a Propensity Score-Matched Analysis
EP: 3.Key Takeaways: The Role of Tazemetostat in Relapsed/Refractory FL Moving Forward
EP: 4.Recap: Use of Tazemetostat in EZH2-Mutant vs Wild-Type Follicular Lymphoma

Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.