Report finds high rate of adoption of IMRT
DES PLAINES, Illinois-Fully 87% of U.S. radiation oncology sites have adopted intensity-modulated radiation therapy (IMRT), according to the “2007 Radiation Oncology Market Summary Report.”
ABSTRACT: The large majority of U.S. radiation oncology sites are now using this therapy.
DES PLAINES, Illinois-Fully 87% of U.S. radiation oncology sites have adopted intensity-modulated radiation therapy (IMRT), according to the “2007 Radiation Oncology Market Summary Report.”
The report, published by the IMV Medical Information Division, is based on profiles of hospital and free-standing radiation oncology sites throughout the country. Findings reflect responses from 652 sites interviewed during an 18-month period (January 2007-June 2008).
Report findings suggested that patients made about 24.5 million visits to 2,110 U.S. radiation oncology sites in 2007-a 5% increase from the 23.2 million visits made in 2006. Of all types of cancer treated with radiation therapy, prostate cancer accounted for 21%, breast cancer accounted for 20%, and lung cancer accounted for 13%.
In terms of treatment planning, 97% of plans used CT, and/or PET imaging. Most (90%) of the simulators installed in 2007 were CT simulators. More than 50% of sites now use image-guided radiation therapy (IGRT), primarily ultrasound, x-ray, and CT, up sharply from 15% in 2004.
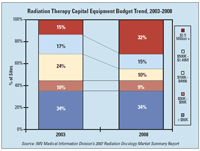
Finally, the percentage of sites having capital budgets of $1.5 million or greater more than doubled between 2003 and 2008, from 15% to 32%.
“While the number of patients treated with radiation therapy every year is relatively stable, the technological sophistication of radiation oncology departments is continuing to advance,” commented Lorna Young, senior director of Market Research at IMV, in a press statement. “Digital imaging has become integrated into treatment planning and to guide tumor treatment real time.”
How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.