Review of Recent Regimensn
ROCHESTER, Minn-Standard adjuvant therapy for stage III colorectal cancer (CRCA) should be 6 months of 5-flourouracil (5-FU) plus either high-dose or low-dose leucovorin (LV), but new agents “offer the promise of less toxicity and possibly improved survival,” Henry C. Pitot, MD, reported. A consultant in the Division of Medical Oncology at the Mayo Clinic in Rochester, Minnesota, Dr. Pitot described several of these new regimens at a clinical investigators’ workshop sponsored by the University of Texas M. D. Anderson Cancer Center and Pharmacia Oncology.
ROCHESTER, MinnStandard adjuvant therapy for stage III colorectal cancer (CRCA) should be 6 months of 5-flourouracil (5-FU) plus either high-dose or low-dose leucovorin (LV), but new agents offer the promise of less toxicity and possibly improved survival, Henry C. Pitot, MD, reported. A consultant in the Division of Medical Oncology at the Mayo Clinic in Rochester, Minnesota, Dr. Pitot described several of these new regimens at a clinical investigators workshop sponsored by the University of Texas M. D. Anderson Cancer Center and Pharmacia Oncology.
Dr. Pitot began with a discussion of a North Central Cancer Treatment Group (NCCTG) study, NCCTG-89-46-51, which found that a two-drug regimen given for 6 months was inferior to a three-drug regimen given for 6 months, and to both two-drug and three-drug regimens given for 12 months. If you are going to treat for 6 months, you need three drugs, but this approach is associated with increased toxicity, he said.
He described an intergroup study, INT-0089, as the trial that killed levamisole as adjuvant therapy for stage III disease. This trial found that levamisole (Ergamisol) added no advantage to 5-FU/LV and that 5-FU/levamisole was inferior to the 5-FU/LV regimens tested.
New Directions
Dr. Pitot said that new directions include modifying the toxicity of treatment by using oral drugs and such new drugs as raltitrexed (Tomudex), combining chemotherapy with irinotecan (Camptosar) or oxaliplatin, and using immunotherapy such as monoclonal antibody MoAb 17-1A or the CeaVac vaccine.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) study NSABP CO-6 has completed accrual of 1,500 patients and will compare 5-FU/high-dose LV for 3 cycles to uracil plus tegafur (UFT) plus oral leucovorin on 28 of 35 days for 5 cycles. This trial may set the stage for a new standard of treatment for stage II-III CRCA, Dr. Pitot said.
Planned accrual for Pan-European Trials in Adjuvant Colon Cancer (PETACC-1) was 2,765 stage III patients, to be randomized either to 6 cycles of 5-FU/HDLV or to 8 cycles of raltitrexed. This trial was prematurely closed to accrual, Dr. Pitot noted, due to excess toxicity in patients randomized to raltitrexed.
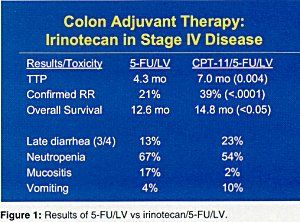
Irinotecan Improves Results
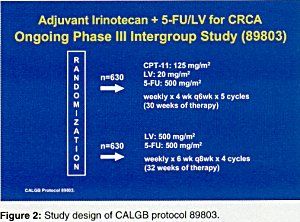
Irinotecan (Camptosar) was tested in stage IV disease, and Dr. Pitot said that toxicity has been manageable and primarily included diarrhea and nausea/vomiting. Adding irinotecan to 5-FU/LV significantly increased the confirmed response rate and time to progression and produced a significant improvement in overall survival (Figure 1). This approach is being tested in stage III disease in Cancer and Leukemia Group B (CALGB) study 89803 (Figure 2).
Oxaliplatin added to 5-FU/LV increased the response rate from 26% to 57% (P < .05) and progression-free survival from 27.8 weeks to 39.6 weeks (P value not significant). Dr. Pitot said that neurosensory toxicity is more common with oxaliplatin but tends to be reversible.
NSABP CO-7 will test 5-FU/HDLV with or without oxaliplatin in 2,472 patients with stage II and III colon cancer. A similar regimen will be tested in 2,000 stage II and III patients in the Multicenter International Study of Oxaliplatin/5-FU in Adjuvant Colon Cancer (MOSAIC).
Immunotherapy Promising
Dr. Pitot said that monoclonal antibody MoAb 17-1A improved 7-year disease-free survival from 32% to 48% compared to a control antibody (P =.04) and 7-year overall survival from 37% to 57% (P < .01) in a small phase III study. Data interpretation was complicated, however, because the trial included patients with rectal cancer and so was not a good test of the antibodys effect in colon cancer alone.
The CeaVac vaccine, which is an anti-idiotype antibody, was promising in a pilot study of 32 patients with resected stage II, III, or IV colon cancer and will be tested further in a proposed American College of Surgeons trial, Dr. Pitot said. All patients generated high-titer IgG and T-cell proliferative immune responses against CEA, and concurrent 5-FU chemotherapy had no effect on immune responses. With regard to clinical outcome, the authors reported, these patients fared remarkably well, Dr. Pitot reported.