Rituximab Combination Is Effective for Relapsed CLL-Associated AIHA
ORLANDO-Patients withchronic lymphocytic leukemia(CLL) complicated by autoimmunehemolytic anemia (AIHA) usuallyimprove following treatment withhigh-dose steroids but have few optionsif such treatment fail
ORLANDO-Patients withchronic lymphocytic leukemia(CLL) complicated by autoimmunehemolytic anemia (AIHA) usuallyimprove following treatment withhigh-dose steroids but have few optionsif such treatment fails.In a poster presented at the 43rdAnnual Meeting of the AmericanSociety of Hematology (abstract1529), Kanti R. Rai, MD, reportedlong-term follow-up data on eightsuch patients suggesting that arituximab (Rituxan), cyclophosphamide,dexamethasone combinationregimen is effective not only as initialsalvage therapy in such patientsbut also as retreatment for relapse.
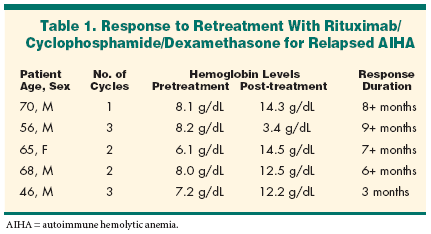
Dr. Rai said that up to 30% ofCLL patients with AIHA either donot respond to conventional steroidregimens or relapse and have a poorresponse to steroid retreatment.Coombs positivity and AIHA areboth well-recognized complicationsof CLL, Dr. Rai said. AIHA arises asa consequence of the destruction ofred blood cells mediated byautoreactive antibodies. The meansby which these autoreactive antibodiesarise is as yet unknown, butit is postulated that the imbalanceof lymphocyte subsets in CLL patientsleads to this complication.Corticosteroids and intravenousimmunoglobulin are the standardtreatment for AIHA. Rituximab isactive in CLL, Dr. Rai said, "so usingrituximab in a patient with CLLcomplicated by AIHA could havetwo potential benefits by eradicatingthe neoplastic CLL cells as wellas achieving control of AIHA."Based on that assumption, Dr.Rai, Niraj Gupta, MD, and their colleaguesat Long Island Jewish MedicalCenter, New Hyde Park, NewYork, studied rituximab, cyclophosphamide,and dexamethasone ineight CLL patients with AIHA refractoryto steroids, intravenousgamma-globulin, or immunosuppressants.Patients were given rituximab375 mg/m2 IV on day 1; cyclophosphamide750 to 1,000 mg/m2 IV onday 2; and dexamethasone 12 mg/dIV on days 1 and 2 and orally ondays 3 to 7. Cycles were repeatedmonthly. Blood products were givenonly to patients with symptomaticanemia or thrombocytopenia.The researchers reported last yearthat all eight patients had an excellentresponse to this regimen. Hemoglobinlevels improved from a pretreatmentmedian of 8.3 g/dL to 13.5g/dL, and Coombs test converted tonegative in five patients after initialtreatment. Duration of initial responsewas 13 months, ranging from7 to more than 23 months.Five of the eight patients subsequentlyrelapsed, and in this posterDr. Rai reported that all five respondedto retreatment with the rituximabcombination (see Table). Hemoglobinimproved from a pretreatmentmedian of 8 g/dL to a post-treatmentmedian of 13.4 g/dL. One patient becameCoombs negative afterretreatment. "All 5 patients remainin response after a median of over 7months," Dr. Rai said.Toxicity included grade 4 neutropeniain one patient, but mostof the side effects were related tothe first rituximab infusion. Nodose modifications were required,and no opportunistic infectionswere observed.The second remissions of AIHAfollowed a median of two cycles of thecombination therapy. Dr. Rai said thatthe improvement in hemoglobin wasevident after the first cycle. Medianduration of response to retreatment is7+ months (range, 3 to 9+ months).One patient had a second relapse after3 months and died 3 months later ofprogressive CLL.Dr. Rai also provided follow-upon the three patients of the originaleight who were not retreated: Twoof these continue in remission at22+ and 23+ months, and one diedof progressive CLL 16 months afterthe initial response to the combinationtherapy.These results demonstrate thatthis regimen is highly effective incontrolling AIHA of CLL in patientswho relapsed after an initial responseachieved earlier with thesame regimen, he said."Such a high success rate wasnot possible to achieve with any ofthe previously used therapies for recurrentAIHA of CLL. Only twocycles were required to achieve themaximum response on retreatment,the toxicity profile is acceptable, andwe think that rituximab deservesexploration in the treatment of thisand other autoimmune hematologicaldisorders," Dr. Rai said."There has been a trend recentlyto give rituximab either in very highdoses weekly or more often thanonce a week. However, these findingsclearly indicate that the standard375 mg/m2 dose is quite effectivewhen given as infrequently asonce a month when combined withother agents such as cyclophosphamideand dexamethasone," Dr. Raiconcluded.