Rituximab Has Significant Activity in Patients With Chronic Lymphocytic Leukemia
Rituximab (Rituxan) is a chimeric monoclonal antibody binding to CD20. A multicenter trial in relapsed low-grade lymphoma (375 mg/m²/wk × 4) produced a response rate of 48%. However, patients with small lymphocytic lymphoma
Rituximab (Rituxan) is a chimeric monoclonal antibody binding to CD20. A multicenter trial in relapsed low-grade lymphoma (375 mg/m²/wk × 4) produced a response rate of 48%. However, patients with small lymphocytic lymphoma (SLL), the tissue equivalent of CLL, had significantly lower response rates (13%) and lower serum levels of rituximab. Reduced response rates in SLL could be related to lower surface expression of CD20 antigen and/or higher circulating B-cell counts. Both factors could also negatively affect response rates in CLL.
In an attempt to maximize the activity of rituximab in CLL, we conducted a phase I/II dose-escalating study. All patients received a first dose of 375 mg/m² to minimize infusion-related side effects. Subsequent weekly doses (three) were given at an increased dose level (fixed for the three doses).
To date, 50 patients have been treated and analyzed. Doses ranged from 500 to 2,250 mg/m². Of the 50 patients, 40 patients had CLL and 10 had related disorders, including mantle cell leukemia (MCL; N = 4), prolymphocytic leukemia (PLL; N = 2), and marginal zone leukemia (MZL; N = 4). Median age was 66 years (range, 44 to 87 years). The majority (80%) of patients had Rai stage 3-4 disease.
Median white blood cell count was 43× 109/L (range, 1 to 334 × 109/L); hemoglobin, 10.7 g/dL (7.4 to 14.7 g/dL); platelet count, 72 × 109/L (9 to 202 × 109/L). Median beta-2-microglobulin was 4.3 mg/L (range, 2.2 to 13 mg/L).
The median number of prior therapies was two (range, zero to six). All patients with CLL had received prior therapy, and 53% were refractory to fludarabine (Fludara).
With the first dose (375 mg/m²), side effects, mainly fever and chills, were seen in all but two patients (94%). Six patients experienced grade 3-4 toxicity, including fever, chills, and dyspnea; five had hypotension, and one had hypertension. Five of these patients with severe toxicity had other diagnoses (with higher surface CD20 expression) and three had high white blood cell counts (229,000, 200,000 and 184,000/µL, respectively).
Almost no toxicity was seen on subsequent escalated doses until the dose reached 2,250 mg/m². No grade 3-4 toxicity was noted, but 67% (8 of 12) of patients had fever, chills, nausea, and fatigue.
The overall response rate was 40%, with 36% of patients with CLL responding. Analysis of response by dose indicated that the lower doses (500 to 825 mg/m²) had a response rate of 23% (6/26), doses between 1,000 and 1,500 mg/m² had a 44% (4/9) response rate, and doses of 2,250 mg/m², an 80% (8/10) response rate.
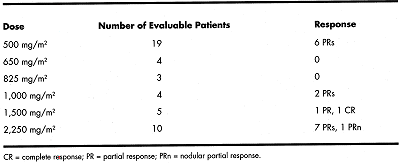
Analysis of response by fludarabine status revealed the response rate in previously sensitive patients was 56% (9/16) vs 20% (4/20) in fludarabine-refractory patients.
CONCLUSION: A correlation between rituximab dose and response is evident, and this antibody has significant activity in the treatment of CLL.
Click here for Dr. Bruce Chesons commentary on this abstract.