Rituximab Plus CHOP May Be New Standard for Treating Aggressive B-cell Lymphomas in the Elderly
LYON, France-In one of the most eagerly awaited presentations at the 42nd Annual Meeting of the American Society of Hematology, Bertrand Coiffier, MD, predicted that the combination of rituximab (Rituxan) plus CHOP "may be the new standard for treatment of aggressive B-cell lymphomas in elderly patients." Head of hematology at Hospices Civilese de Lyon, Lyon, France, Dr. Coiffier presented an interim analysis of results from a European phase III trial of rituximab plus CHOP (cyclophosphamide, doxorubicin, Oncovin [vincristine], prednisone) in elderly patients with diffuse large B-cell lymphomas (DLBCL).
LYON, FranceIn one of the most eagerly awaited presentations at the 42nd Annual Meeting of the American Society of Hematology, Bertrand Coiffier, MD, predicted that the combination of rituximab (Rituxan) plus CHOP "may be the new standard for treatment of aggressive B-cell lymphomas in elderly patients." Head of hematology at Hospices Civilese de Lyon, Lyon, France, Dr. Coiffier presented an interim analysis of results from a European phase III trial of rituximab plus CHOP (cyclophosphamide, doxorubicin, Oncovin [vincristine], prednisone) in elderly patients with diffuse large B-cell lymphomas (DLBCL).
The Groupe d’Etude des Lymphomas de l’Adulte (GELA) study compared classic CHOP with CHOP plus the anti-CD20 monoclonal rituximab. The study included more than 130 centers in France, Belgium, and Switzerland.
Patient Accrual Was Quick
The open-label GELA trial enrolled 400 previously untreated elderly patients (aged 60 to 80) with stage II to IV DLBCL who had no contraindication to doxorubicin. "We had planned to recruit 400 patients over 3 years but got all of them in 18 months," Dr. Coiffier said.
Patients were stratified for age-adjusted International Prognostic Index (IPI) scores and randomized either to standard CHOP every 3 weeks for 8 cycles or to standard CHOP plus rituximab at 375 mg/m2 on day 1 of each cycle (R-CHOP). The primary endpoint was event-free survival (EFS), with events defined as disease progression or relapse, death, or initiation of different treatment. Secondary endpoints were survival, response rate, and safety.
An interim analysis was prospectively planned when the first 100 patients had been followed for 1 year, but accrual was so quick that the investigators decided to include all 328 evaluable patients in the interim report. Median follow-up for this group was 1 year.
Responses Higher With R-CHOP
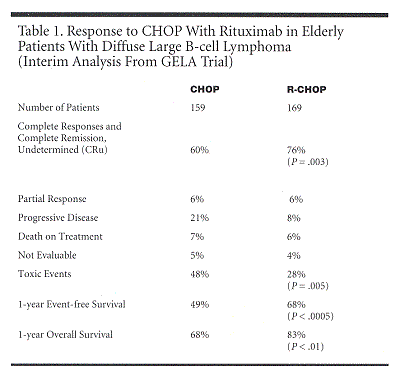
The interim analysis included 159 patients randomized to CHOP and 169 randomized to R-CHOP. Adverse prognostic parameters were equally distributed between arms: 63% of patients had stage IV disease, 19% had PS > 1, 65% had elevated LDH, 26% had bone marrow involvement, 52% had > 1 extranodal disease sites, and 60% had IPI = 2 or 3. Complete responses were significantly higher with R-CHOP than with classic CHOP (see Table 1). "At 1 year, there was a 20% difference in event-free survival between the two arms and a 15% difference in overall survival. These were highly statistically significant," Dr. Coiffier said.
Toxicity was not increased by the addition of rituximab to chemotherapy, Dr. Coiffier said. "In fact, we observed fewer grade 3-4 events with R-CHOP than with classic CHOP." There were 20 cardiac toxicity events in the 159 CHOP patients vs 21 in the 169 R-CHOP patients.
Patient withdrawals due to toxicity were similar in the two arms (13 with R-CHOP vs 17 with CHOP), but more patients withdrew from the CHOP arm than from the R-CHOP arm due to treatment failure (18 vs 5). Toxic events were significantly greater with CHOP than with R-CHOP for unexplained reasons. Dr. Coiffier reported that treatment failures usually occurred after the fourth cycle of treatment, but that patients in both arms nonetheless received the same median dose of CHOP.
Oliver Press, MD, PhD, introduced the presentation as "a landmark study" and pointed out the clinical importance of this trial: diffuse large B-cell lymphomas account for 31% of non-Hodgkin’s lymphomas and 17,000 new cancer cases per year in the United States. Dr. Press is professor of medicine at the University of Washington.
"In patients under age 64, 35% to 40% can be cured with CHOP, but older patients are less likely to benefit," Dr. Press said. "Five-year survival after CHOP is 51% for patients under age 60 vs 38% for patients 60 or older. Patients over 60 are also less likely to be eligible for high-dose chemotherapy with stem cell transplant, a common approach for treating relapse in younger patients."
Confirmatory Trial Underway
Dr. Press raised several cautionary points about the GELA data, most importantly that this is an interim analysis including 328 of 400 patients with a relatively short (11.5 months) follow-up. He said that a confirmatory trial is needed and fortunately, one is underway. This Intergroup study (E4494) has enrolled about 500 patients and is expected to complete accrual in early 2001.
"It is also important not to extrapolate the GELA results to other B-cell lymphomas such as mantle cell and follicular lymphomas," Dr. Press said.
During the discussion period Sandra Horning, MD, senior investigator for the E4494 study and professor of medicine, Stanford University, revealed that results of the GELA study had inspired the Eastern Cooperative Oncology Group (ECOG) monitoring committee to take an unplanned look at the E4494 data.
Dr. Horning said that the monitoring committee recommended that the study continue, an indication that any survival difference (or toxicity) observed thus far in the intergroup study was not sufficient to justify stopping the study.
Therefore, E4494, a study that includes a second randomization to observation or rituximab maintenance, will continue to accrue patients on the recommendation of the Data Monitoring Committee.