The Role of LHRH Antagonists in the Treatment of Prostate Cancer
Physicians have known since 1941 that testosterone suppression benefits patients with symptomatic metastatic prostate cancer.[1] The pioneering study in this regard showed that estrogen therapy achieved comparable efficacy to castration by improving acid and alkaline phosphatase levels associated with relief of cancer-related symptoms. More than 6 decades later, however, many of the therapies subsequently developed for achieving androgen deprivation still suffer from serious limitations.
ABSTRACT: For patients with advanced prostate cancer, luteinizing hormone-releasing hormone (LHRH) agonists have provided successful androgen deprivation therapy (ADT) for some 25 years. However, the benefits of LHRH agonists are limited in that these agents are agonists, not antagonists. The search for and development of an effective LHRH antagonist have proven difficult. Nevertheless, antagonists offer subtle advantages, including more rapid reduction in testosterone levels, reduction in testosterone-induced flare, and maintenance of castrate levels of testosterone. Accordingly, LHRH antagonists appear to provide a viable alternative to LHRH agonist therapy. Degarelix, a recently approved LHRH antagonist, has been shown to work more quickly in lowering serum testosterone levels, with an acceptable safety profile and a mechanism of action that obviates the testosterone surges associated with LHRH agonist use. Presently, degarelix is the only LHRH antagonist approved for the treatment of advanced prostate cancer.
Physicians have known since 1941 that testosterone suppression benefits patients with symptomatic metastatic prostate cancer.[1] The pioneering study in this regard showed that estrogen therapy achieved comparable efficacy to castration by improving acid and alkaline phosphatase levels associated with relief of cancer-related symptoms. More than 6 decades later, however, many of the therapies subsequently developed for achieving androgen deprivation still suffer from serious limitations.
Although effective, estrogen therapy carried significant cardiovascular toxicity that severely limited its utility. Specifically, a series of Veterans Administration Cooperative Urological Research Group studies raised serious concerns regarding cardiac morbidity and mortality in patients receiving diethylstilbestrol (DES).[2,3] The most commonly used estrogenic agent for achieving medical castration, DES inhibits luteinizing hormone-releasing hormone (LHRH) through negative feedback on the hypothalamus-pituitary axis.
In the 1960s and 1970s, research into antiandrogenic compounds culminated in the development of several agents that block the testosterone receptor while producing minimal side effects.[4,5] Although monotherapy with steroidal antiandrogens, including cyproterone acetate, megestrol acetate, and medroxyprogesterone acetate, produce side effects including impotence and libido loss, nonsteroidal antiandrogens such as flutamide, nilutamide (Nilandron), and bicalutamide (Casodex) avoid these problems because they act only at the androgenic receptors. Nevertheless, oncologists generally do not consider antiandrogens to be an optimal choice for metastatic prostate cancer because these agents are perhaps less effective than both orchiectomy and the therapeutic class that superseded antiandrogens, LHRH agonists.[6]
Advantages and Disadvantages of LHRH Agonists
Introduced in the United States in 1984,[7] LHRH agonists essentially deplete the pituitary hormone and downregulate pituitary LHRH receptors, rendering the pituitary nonresponsive to further stimulation by LHRH. Currently available LHRH agonists include leuprolide, goserelin (Zoladex), buserelin, and triptorelin (Trelstar). Available in various formulations, these drugs are administered via depot injections that last as long as 12 months.
However, disadvantages of LHRH agonists include high repeating costs, libido loss, impotence, and hot flashes. Importantly, the testosterone surge that occurs after initial injection of LHRH agonists can cause tumor flare in up to 63% of patients with advanced disease.[8] In certain men with advanced prostate cancer, these flares are accompanied by pain and serious side effects that can include urethral obstruction and spinal cord compression, which can lead to paralysis and, rarely, death.[9] Accordingly, oncologists frequently administer short-term or long-term antiandrogens to prevent testosterone surge in patients taking LHRH agonists.
Introduction of LHRH Antagonists
Unlike LHRH agonists, LHRH antagonists (also known as gonadotropin-releasing hormone [GnRH] antagonists), avoid testosterone surge.[10,11] Moreover, they achieve castrate serum testosterone levels much more quickly than the 30 days LHRH agonists typically require to achieve this goal.[12] The first LHRH antagonist, abarelix (Plenaxis), was approved by the US Food and Drug Administration (FDA) for advanced, symptomatic prostate cancer in late 2003. Phase III clinical trials showed that abarelix monotherapy achieved medical castration more quickly than leuprolide alone[10] or combined with bicalutamide. Furthermore, these studies showed that abarelix suppresses follicle-stimulating hormone (FSH), a possible growth stimulator for prostate cancer cells.[10,11,13]
In a phase II trial, abarelix depot provided not only rapid testosterone suppression, but also a higher rate of medical castration than did LHRH agonists with or without antiandrogens.[14,15] Other early testing showed that adverse events following abarelix administration were primarily limited to those associated with prostate cancer itself and patient age.[16] In clinical practice, however, abarelix’s propensity to cause histamine release gave rise to potentially life-threatening, immediate-onset systemic allergic reactions.[17] The FDA also warned that abarelix could stop working for some patients. Thus, its manufacturer ceased marketing the product in the United States in 2005.
The failure of abarelix mirrors the fate of several other LHRH antagonists studied for treating prostate cancer. Many never reached clinical development because along with histamine-releasing properties, these agents tended to possess solubility limitations and/or an inability to sustain clinical efficacy.[18] In fact, according to abarelix labeling, the product maintained serum testosterone levels below 0.5 ng/mL in only 62% to 71% of patients after 1 year’s use.[17]
A More Effective Alternative
Degarelix-which was FDA-approved for advanced prostate cancer in late 2008-has demonstrated the ability to provide rapid, profound, sustained suppression of testosterone without testosterone surges. That is because rather than binding to the pituitary LHRH receptor and initially activating receptors that could lead to a disease flare, LHRH antagonists block the receptor, suppressing release of gonadotropins and testosterone.[19] Moreover, unlike abarelix, degarelix-a synthetic peptide used by more than 2,000 patients during clinical development-has shown no signs of contributing to immediate- or late-onset systemic allergic reactions.[20]
In phase II dose-ranging studies of degarelix at a starting dose of 240 mg and maintenance doses of 80 or 160 mg, the drug effectively suppressed testosterone levels in patients with prostate cancer, with no evidence of testosterone surge or clinical flare.[19,20]
Phase III Degarelix Trial
In a pivotal phase III clinical study, two doses of degarelix proved to be as effective as a typical 7.5-mg dose of leuprolide in achieving and maintaining testosterone suppression for 1 year.[21] For this active-controlled, open-label trial, investigators randomized 610 patients into three groups. Specifically, all patients treated with degarelix received a 240-mg starting dose, followed by monthly (every 28 days) maintenance doses of either 80 mg (207 men) or 160 mg (202 men). Patients in the leuprolide cohort (201 men) received 12 monthly intramuscular injections of leuprolide, 7.5 mg. At the investigators’ discretion, 23 patients in the leuprolide group also took bicalutamide (50 mg, once daily) at the start of treatment to protect against clinical flares.
The study population included men at least 18 years old with histologically confirmed prostate cancer of any stage whom investigators considered appropriate candidates for endocrine treatment (except for neoadjuvant hormonal therapy). As such, the population included patients with biochemical failure and hormone-sensitive metastatic disease-patients whose prostate-specific antigen (PSA) levels were increasing despite treatment with curative intent. In particular, researchers selected patients with PSA levels ≥ 2 ng/mL, as well as screening serum testosterone levels > 1.5 ng/mL, and Eastern Cooperative Oncology Group scores ≤ 2. However, investigators excluded patients with any history of hormonal management therapies, unless it was localized therapy of curative intent in which they used neoadjuvant or adjuvant hormonal therapy for no more than 6 months and discontinued this treatment at least 6 months prior to study enrollment. Investigators also excluded patients considered candidates for curative therapy.
All three treatment groups possessed comparable baseline demographics and characteristics. Median patient age, for example, was 73 years (range: 50–98), median PSA level was 19.0 ng/mL (range: 8.7–57), and median serum testosterone level was 3.93 ng/mL (range: 2.89–5.10). As for disease stage, 31% of patients had localized disease, 29% locally advanced, 20% metastatic, and 19% unclassifiable.
• Study Results-The primary endpoint of the trial was suppression of testosterone to ≤ 0.5 ng/mL at all monthly measurements from days 28 to 364. Statistical analysis of this endpoint for degarelix yielded a lower limit of the 95% confidence interval that was consistently ≥ 90%. Furthermore, degarelix proved at least as effective as leuprolide in maintaining treatment response throughout this time period. In the 240/80 mg and 240/160 mg degarelix treatment groups, 97.2% and 99.4% of patients, respectively, responded to treatment. By comparison, 96.3% of leuprolide-treated patients achieved a response. However, 15 patients (a total of 8 treated with degarelix and 7 with leuprolide) had at least one testosterone value > 0.5 ng/mL between days 28 and 364. Researchers defined two consecutive testosterone values at this level-or one value > 1.0 ng/mL-as an insufficient response, which was experienced by four patients who took degarelix at 240/80 mg, two patients who took degarelix at 240/160 mg, and six who took leuprolide.
FIGURE 1

Testosterone Reduction
Degarelix also provided rapid suppression of testosterone levels (Figure 1).[22] By day 3, 96.1% and 95.5% of patients in the degarelix 240/80 and 240/160 mg groups, respectively, achieved median testosterone levels ≤ 0.5 ng/mL. Conversely, patients taking leuprolide experienced a median testosterone increase of 65% over baseline by day 3 (P < .001, degarelix vs leuprolide). It took until day 28 for median testosterone levels in this group to reach 0.5 ng/mL or less. After day 28, all three treatments maintained testosterone suppression through the study’s end.
The concomitant bicalutamide taken by 23 patients on leuprolide had little effect in preventing testosterone surges. In this group, 74% of patients experienced a testosterone surge, vs 81% who took leuprolide without bicalutamide. No testosterone surges were seen in either degarelix treatment group.
While treatment with degarelix resulted in rapid decreases in median LH and FSH values, these levels increased early in the study for patients who took leuprolide. FSH levels in the leuprolide group never fell as low as they did in both degarelix groups. By study’s end, mean FSH levels in the degarelix 240/80 mg, degarelix 240/160 mg, and leuprolide groups had decreased by 88.5%, 89.0%, and 54.8% , respectively. Persistent FSH suppression is seen with GnRH antagonist therapy, but the therapeutic advantage of this suppression is unclear.[23] Research by Sun et al demonstrated that FSH stimulates osteoclastogenesis and bone resorption. The suppression of FSH may reduce the risk of osteoporosis with prolonged GnRH analog treatment.[24]
FIGURE 2

PSA Reduction
Additionally, degarelix provided rapid reduction in PSA levels (Figure 2).[22] After day 14, PSA levels had fallen 64% and 65% from baseline in the degarelix 240/80 mg and 240/160 mg groups, respectively, vs 18% in the leuprolide group. The corresponding figures after 28 days were 85%, 83%, and 68%, respectively.
Differences in PSA reduction from baseline between degarelix and leuprolide were statistically significant at both time points (P < .001). Patients who took leuprolide and bicalutamide experienced PSA reductions similar to those achieved by degarelix. Due to the heterogeneity of the patient population, however, one must interpret PSA findings cautiously. No evidence has shown that a faster decline in PSA confers any clinical benefit.[21]
The investigators defined PSA failure as two consecutive PSA increases of > 50% from nadir and ≥ 5 ng/mL on two consecutive occasions at least 2 weeks apart. The incidence of PSA failure was similar among all study groups: 8.9% for degarelix at 240/80 mg, 14.2% for degarelix at 240/160 mg, and 14.1% for leuprolide.
TABLE 1
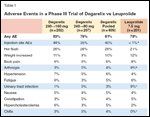
Adverse Events in a Phase III Trial of Degarelix vs Leuprolide
• Side-Effect Profiles-Adverse events (AEs) occurred with similar frequency in all three treatment groups (Table 1). Investigators reported treatment-emergent AEs for 79% of patients in the degarelix 240/80 mg group, 83% of the degarelix 240/160 mg group, and 78% of the leuprolide group. Most AEs were mild to moderate in severity, with the most common AE being flushing, which was reported by 26% of patients in each degarelix group and 21% in the leuprolide group. The subcutaneous degarelix injections produced higher rates of injection-site reactions (usually occurring upon initial dosing) and chills than the intramuscular leuprolide injections did, whereas patients who took leuprolide experienced more arthralgia and urinary tract infections than did patients who took degarelix.
Along with injection site reactions (which occurred in 35% of patients taking degarelix), the most common AEs reported with degarelix use included hot flashes, weight gain, fatigue, and increases in serum levels of transaminases and gamma-glutamyltransferase. Investigators reported no systemic allergic reactions to degarelix, while such reactions were a concern with abarelix. Serious AEs occurred in 10% and 12% of patients in the degarelix 240/80 mg and 240/160 mg cohorts, respectively, vs 14% in the leuprolide cohort. No patient who took degarelix experienced a serious AE that investigators considered related to treatment, and fewer deaths occurred among degarelix patients (5 per cohort) than leuprolide patients (9).
Prognosis for Degarelix
Overall, the quicker onset of action and lack of testosterone surge associated with degarelix therapy have apparent clinical value for patients with advanced disease who have or are at risk of having prostate cancer symptoms, and for those planned for short-term adjuvant, neoadjuvant, or intermittent treatment. “It remains to be established. . . whether the faster onset of action, the absence of surges and microsurges, the more effective FSH suppression, the possibility of a further different safety profile, or any hitherto unmeasured difference between agonist and blocker provide further clinical value,” the phase III study’s authors wrote.[21]
Moreover, the manufacturer of degarelix (Ferring Pharmaceuticals) states that upon contact with subcutaneous tissue, elements within the degarelix suspension immediately form a gel-like depot that ensures gradual release of the drug over 28 days. As such, the drug requires no added ingredients to control its release. It must be noted, however, that LHRH agonist formulations typically allow these injections to last 3 to 4 months and, in some cases, up to 1 year.
Nevertheless, phase III research establishes that degarelix represents a safe, effective new therapy for achieving and maintaining androgen deprivation for 1 year in patients with prostate cancer, which is the second-leading cause of US cancer deaths in men.[25] The research shows that the rapid onset of action of degarelix suppresses testosterone, PSA, and FSH levels more quickly than leuprolide does, while degarelix eliminates the need for flare prophylaxis associated with LHRH agonists and avoids some of the risks associated with its predecessor, abarelix.
Currently, the drug is only available as a monthly injection, which can be perceived as a challenge. In our clinical practice, the majority of LHRH agonist usage involves the longer-acting depot preparations. It is anticipated that a longer-acting depot preparation of the new LHRH antagonist will be forthcoming.
Financial Disclosure: Dr. Crawford serves on speakers bureaus for Ferring, Watson, AstraZeneca, GlaxoSmithKline, and Indevus, and advisory boards for Ferring and Indevus. Dr. Hou reports no relevant financial interests.
References:
1. Huggins C, Hodges CV: Studies on prostate cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1:293, 1941.
2. Veterans Administration Cooperative Urological Research Group: Treatment and survival of patients with cancer of the prostate. Surg Gynecol Obstet 124:1011, 1967.
3. Byar DP, Corle DK: Hormone therapy for prostate cancer: Results of the Veterans Administration Cooperative Urological Research Group studies. Natl Cancer Inst Monogr 7:165, 1988.
4. Kalsary AV, Tyrrell CJ, Beacock C, et al: A randomised comparison of monotherapy with Casodex 50 mg daily and castration in the treatment of metastatic prostate carcinoma. Casodex Study Group. Eur Urol 28:215-222, 1995.
5. Iversen P, Tveter K, Varenhorst E: Randomised study of Casodex 50 mg monotherapy vs orchidectomy in the treatment of metastatic prostate cancer. The Scandinavian Casodex Cooperative Group. Scand J Urol Nephrol 30:93-98, 1996.
6. Schmitt B, Wilt TJ, Schellhammer PF, et al: Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: A systematic review. Urology 57:727-732, 2001.
7. Moul JW: Twenty-five year evolution of medical hormonal therapy for prostate cancer. BJU Int 103:145-146, 2009.
8. Labrie F, Dupont A, Belanger A, et al: Flutamide eliminates the risk of disease flare in prostatic cancer patients treated with a luteinizing hormone-releasing hormone agonist. J Urol 138:804-806, 1987.
9. el-Rayes BF, Hussain MH: Hormonal therapy for prostate cancer: Past, present and future. Expert Rev Anticancer Ther 2:37-47, 2002.
10. MacLeod DG, Zinner N, Tomera K, et al: A phase 3, multicenter, open-label randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology 61(suppl 1):3-7, 2003.
11. Trachtenberg J, Gittleman M, Steidle C, et al: A phase 3, multicenter, open-label randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol 167:1670-1674, 2002.
12. Lupron Depot 7.5 mg (package insert). Lake Forest, Ill; TAP Pharmaceuticals Inc; 2007.
13. Beer TM, Garzotto M, Eilers KM, et al: Targeting FSH in androgen-independent prostate cancer: Abarelix for prostate cancer progressing after orchiectomy. Urology 63:342-347, 2004.
14. Debruyne FM: Gonadotropin-releasing hormone antagonist in the management of prostate cancer. Rev Urol 6(suppl 7):S25-S32, 2004.
15. Tomera K, Gleason D, Gittelman M, et al: The gonadotropin-releasing hormone antagonist abarelix depot versus luteinizing hormone releasing hormone agonists leuprolide or goserelin: Initial results of endocrinological and biochemical efficacies in patients with prostate cancer. J Urol 165:1585-1589, 2001.
16. Garnick MB: Novel treatments for advanced prostate cancer: The new paradigm. Presented at 10th International Prostate Cancer Update; Vail, Colo; Feb 2–6, 2000.
17. US Food and Drug Administration: Questions and answers about Plenaxis (abarelix for injectable suspension). Available at www.fda.gov/Cder/Drug/infopage/plenaxis/plenaxisQ&A.htm. Accessed March 17, 2009.
18. Huirne JAF, Lambalk CB: Gonadotropin-releasing-hormone-receptor antagonists. Lancet 358:1793-1803, 2001.
19. Van Poppel H, Tombal B, de la Rosette JM, et al: Degarelix: A novel gonadotropin-releasing hormone (GnRH) blocker-results from a one-year, multicentre, randomised, phase 2 dose-finding study in the treatment of prostate cancer. Eur Urol 54:805-815, 2008.
20. Gittelman M, Pommerville PJ, Persson BE, et al: A 1-year, open-label, randomized phase II dose-finding study of degarelix, a novel gonadotropin-releasing hormone (GnRH) receptor blocker, in the treatment of prostate cancer in North America. J Urol 180:1986-1992, 2008.
21. Klotz L, Boccon-Gibod L, Shore ND, et al: The efficacy and safety of degarelix: A 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 102:1531-1538, 2008.
22. Boccon-Gibod L, Klotz L, Schröder H, et al: Degarelix compared to leuprolide depot 7.5 mg in a 12-month randomised, open-label, parallel-group phase III study in prostate cancer patients (abstract 537). Presented at the 23rd Annual EAU Congress, Milan, Italy, 2008.
23. Huhtaniemi I, White R, McArdle C, et al: Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Metab 20:43-50, 2009.
24. Sun L, Peng YZ, Sharrow AC, et al: FSH directly regulates bone mass. Cell 125:247-260, 2006.
25. American Cancer Society: How many men get prostate cancer? Available at http://www.cancer.org/. Accessed March 20, 2009.