Safety of Fludarabine, Mitoxantrone, and Dexamethasone Plus Rituximab in the Treatment of Stage IV Indolent Lymphoma
A total of 77 patients have been entered into an ongoing, randomized protocol integrating rituximab (Rituxan) with chemotherapy for patients with newly diagnosed stage IV indolent lymphoma. Patients are receiving FND (fludarabine
A total of 77 patients have been entered into an ongoing, randomized protocol integrating rituximab (Rituxan) with chemotherapy for patients with newly diagnosed stage IV indolent lymphoma. Patients are receiving FND (fludarabine, Novantrone, and dexamethasone) concurrently with rituximab in one arm and FND followed by rituximab in the other arm. All patients have received prophylaxis for Pneumocystis infection. A subset of patientsthose with follicular lymphoma but no detectable bcl-2 gene rearrangementare considered prognostically adverse (Lopez-Guillermo et al: Blood 93:308-3087, 1999) and are being managed with an alternating triple therapy (ATT) 10-drug regimen (Ann Oncol 5(suppl 2):S73, 1994).
Preliminary safety data are as follows:
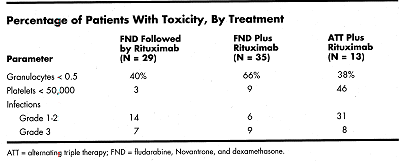
There have been no grade 4 infections or nonhematologic toxicities. The six grade 3 infections included two catheter-related bacteremias, two other bacterial infections, and two fevers of unknown origin that responded to antibacterial therapy. There have been no unusual opportunistic infections; there has been one episode of localized herpes zoster.
CONCLUSION: It appears that the combination of FND and rituximab, administered either concurrently or sequentially, is a safe regimen.
Click here for Dr. Bruce Chesons commentary on this abstract.