Skin cancer is the most commonly diagnosed cancer in the United States and has become a major public health problem that continues to grow. Despite numerous initiatives and increased public awareness of the dangers of skin cancer, the incidence of skin cancer continues to rise worldwide. This highlights the need for a critical review of existing skin cancer prevention strategies as a means to determine where targeted efforts may improve patient outcomes. In this article, we review the published literature and evaluate secondary prevention strategies for nonmelanoma skin cancer. Specifically, we examine the existing data on the use of chemopreventive agents for nonmelanoma skin cancer primarily in immunocompetent individuals, but also in organ transplant recipients, the best-studied immunosuppressed population. We also explore investigational therapies proposed for chemoprevention of nonmelanoma skin cancers.
Introduction
The incidence of skin cancer is increasing worldwide and has become a serious public health concern.[1] Skin cancer can be classified as either nonmelanoma skin cancer-which includes basal cell carcinoma (BCC), squamous cell carcinoma (SCC), keratoacanthoma, angiosarcoma, Merkel cell carcinoma, cutaneous B- and T-cell lymphoma, dermatofibrosarcoma protuberans, and sebaceous gland carcinoma-or as melanoma. Nonmelanoma skin cancers represent the majority of cutaneous malignancies and affect one in five Americans during their lifetime.[1] While the prognosis for most patients is excellent, significant morbidity may occur due to the predilection of these cancers for arising in cosmetically sensitive areas such as the head and neck.[2–5]
Skin cancer is largely preventable; thus, implementation of both primary and secondary prevention strategies can profoundly affect the incidence of these diseases. Primary prevention strategies focus on reduction of risk factors via environmental and behavioral modification. While the goal of primary prevention is to prevent disease from developing, secondary prevention aims at detecting and controlling cancerous or precancerous processes while disease is localized.[6] Here, we review the published literature regarding chemoprevention for secondary prevention of skin cancer, focusing on chemoprevention of nonmelanoma skin cancers primarily in immunocompetent individuals. We also review data on chemoprevention in organ transplant recipients, the best-studied immunosuppressed population.
Secondary Prevention: What Is It, and Why Is It Important?
Ultraviolet (UV) radiation is the most preventable cause of skin cancer.[7] Primary prevention efforts have focused on increasing awareness about the dangers of excessive UV exposure.[8–10] The association between UV exposure and nonmelanoma skin cancer suggests that interventions rooted in sun safety would be effective in decreasing skin cancer incidence.[11–17] Despite this, many individuals do not adhere to recommended guidelines to protect themselves from UV exposure,[18] necessitating increased emphasis on secondary prevention strategies.
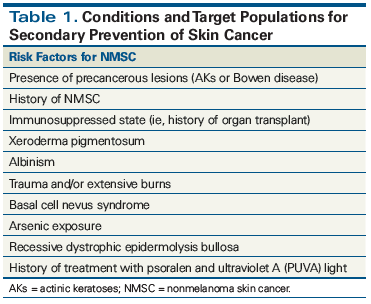
Secondary prevention focuses on early detection and control of cancer, thereby leading to timely initiation of therapy and improved outcomes, and is critically important for patients at high risk for nonmelanoma skin cancer (Table 1). Screening tests and/or exams are the cornerstones of secondary prevention strategies. By identifying individuals with particular risk factors, screening tests allow for the detection of disease in asymptomatic individuals.
Skin cancer screening
While secondary prevention strategies are well established for malignancies such as breast and colon cancer, the lack of clear skin cancer screening guidelines has hindered secondary prevention initiatives.[19] The US Preventive Services Task Force concluded that there was not enough evidence to recommend routine skin cancer screening. However, key considerations were missing from their analysis, including the morbidity associated with delayed diagnosis of nonmelanoma skin cancers.[20] Though studies conducted outside the United States have demonstrated that skin cancer screening can decrease melanoma mortality, no rigorous studies have examined its utility for early detection of nonmelanoma skin cancers.[21–24]
Chemoprevention as a secondary prevention strategy
Chemoprevention involves the use of chemicals and biologic agents to prevent or delay the development of cancer. In addition to skin cancer screening, secondary prevention strategies for skin cancer also include chemoprevention. Primary chemoprevention is generally understood to be therapy directed toward high-risk patients with the intent of preventing initial carcinogenesis. Secondary chemoprevention is targeted at patients with a history of skin cancer and is undertaken with the goal of preventing disease recurrence.
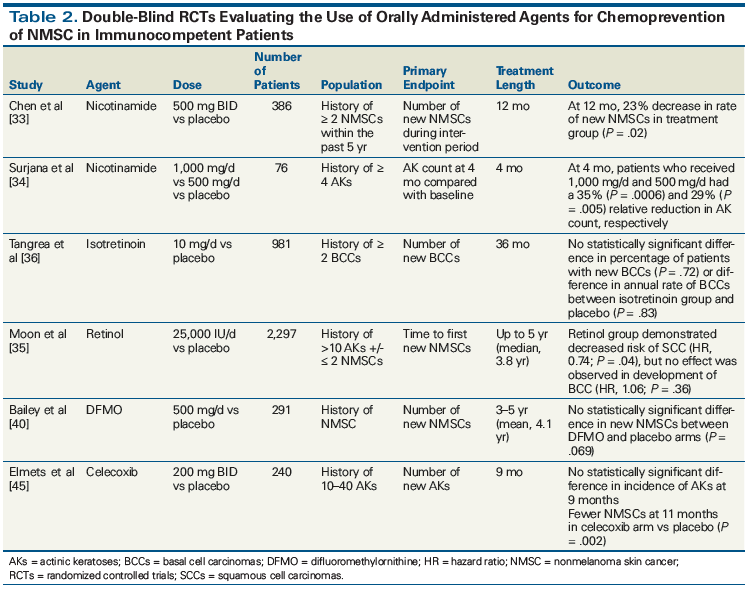
While agents such as vitamin E, betacarotene, and selenium did not demonstrate efficacy in the prevention of nonmelanoma skin cancer, efficacy has been observed with other agents (Table 2).[25-30] In evaluating potential chemopreventive agents for nonmelanoma skin cancer, we have elected to assess randomized controlled trials (RCTs) only. Importantly, each of these trials enrolled only immunocompetent patients. Nonmelanoma skin cancers developing in the setting of immunosuppression differ clinically and biologically from those arising in immunocompetent patients[31]; thus, the applicability of these results to patients with HIV or those on long-term immunosuppression is unclear.
Nicotinamide. Nicotinamide is a water-soluble form of vitamin B3 (niacin) and is the precursor of nicotinamide adenine dinucleotide (NAD+), an essential cofactor for adenosine triphosphate production.[32] Recently, the use of nicotinamide has demonstrated efficacy in the prevention of nonmelanoma skin cancer in high-risk patients. In a phase III double-blind RCT, 386 patients who had had 2 or more nonmelanoma skin cancers within the past 5 years were randomly assigned to receive 500 mg of nicotinamide twice daily or placebo for 12 months.[33] The primary endpoint was the number of new nonmelanoma skin cancers during the 12-month intervention period, and secondary endpoints included the number of new actinic keratoses (AKs)-premalignant cutaneous lesions-during the intervention period, as well as the number of new nonmelanoma skin cancers in the 6-month post-intervention period. At 12 months, the rate of new nonmelanoma skin cancers dropped by 23% in the nicotinamide group compared with the placebo group (P = .02). The number of new AKs was lower in the nicotinamide arm by 11% at 3 months (P = .01), 14% at 6 months (P < .01), 20% at 9 months (P < .001), and 13% at 12 months (P = .001). There were no significant differences in types or number of adverse events between groups during the 12-month intervention period. After discontinuation of nicotinamide, there was no evidence of lasting benefit.[33]
In another study, 76 patients were randomized to take 500 mg of nicotinamide or placebo either once or twice daily for 4 months.[34] Patients eligible for enrollment were healthy, immunocompetent adults with ≥ 4 palpable AKs on the head and upper limbs. The primary endpoint of this study was AK count at 4 months. At 4 months, there was a 35% reduction in AK count in the nicotinamide 1,000 mg/d dosing arm compared with placebo (P = .0006). In the group that received nicotinamide 500 mg/d, there was a 29% reduction in AK count compared with placebo (P = .005). There was no evidence to suggest that the data were falsely skewed, given the significant variation in baseline AK count. During the 4-month trial, 20 new nonmelanoma skin cancers were diagnosed in 11 patients assigned to the placebo arm, while only 2 patients in either of the nicotinamide dosing groups developed a new skin cancer. Odds ratio (OR) analysis demonstrated that the odds of developing at least one skin cancer were significantly lower with nicotinamide (OR, 0.14; 95% CI, 0.03–0.73; P = .019). After adjusting for number of previous skin cancers, logistical regression models demonstrated that the rate of new nonmelanoma skin cancers was also significantly lower in the nicotinamide arms compared with placebo (relative rate, 0.24; 95% CI, 0.08–0.71; P = .010).[34] Spontaneous regression of AKs was considered during analysis and reflected in the placebo groups. No significant side effects were reported, and no clinically significant changes in blood chemistries were observed.
Retinoids. Retinoids are a group of chemical compounds derived from vitamin A, with the capacity to modulate cell proliferation, differentiation, apoptosis, and other critical cellular functions. Data regarding the efficacy of retinoid supplementation for the prevention of nonmelanoma skin cancer are somewhat variable. In a double-blind RCT, 2,297 moderate-risk patients with a history of 10 or more AKs and 2 or fewer SCCs/BCCs were assigned to receive either 25,000 IU of oral retinol daily or placebo for up to 5 years.[35] The number of first/new BCCs or SCCs were primary endpoints. After a median follow-up time of 3.8 years, retinol was shown to significantly decrease the risk of SCC (P = .04); however, there was no effect on the risk of new BCC (P = .36). Adverse events such as increased total serum cholesterol levels, increased aspartate transaminase/alanine transaminase levels, mucocutaneous reactions, arthralgia/myalgia, and headaches were 1% higher in the treatment group compared with placebo.[35] Likewise, another study demonstrated that long-term therapy with low-dose isotretinoin in patients with a history of 2 or more BCCs showed no statistically significant difference in prevention of BCCs when compared with placebo.[36]
The use of retinol and isotretinoin has also been explored in high-risk populations. In a double-blind placebo-controlled RCT, patients with a history of 4 or more BCCs and/or SCCs were randomized to receive 25,000 IU of oral retinol, 5–10 mg of isotretinoin, or placebo daily for 3 years.[37] Time to first new nonmelanoma skin cancer was the primary endpoint. At study completion, there was no difference in the time to development of new nonmelanoma skin cancer or total number of new cases between the retinol, isotretinoin, and placebo groups.[37]
Difluoromethylornithine (DFMO). DFMO is an irreversible inhibitor of ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis. UV radiation has been shown to upregulate the activity of ODC, and increased cellular levels of polyamines have been implicated in epithelial carcinogenesis.[38,39] In one study, 291 individuals with a history of nonmelanoma skin cancer received oral DFMO (500 mg/m2/d) or placebo for 4 to 5 years.[40] The primary endpoint, development of new nonmelanoma skin cancer, was not significantly different between the two arms (P = .069). When development of BCC and SCC were analyzed independently, there was a significant decrease in the development of BCCs in the treatment arm compared with placebo (0.28 BCC/person/year vs 0.40 BCC/person/year; P = .03). In a follow-up study, retrospective analysis did not show a “rebound” increase in the rate of BCC or SCC after discontinuation of DFMO. No long-term adverse events were noted.[41]
Celecoxib. Celecoxib is a nonsteroidal anti-inflammatory drug that selectively inhibits cyclooxygenase-2 (COX-2), an enzyme involved in prostaglandin synthesis. In light of evidence implicating UV-induced prostaglandin synthesis as a potential mechanism underlying skin photocarcinogenesis, COX-2 inhibition has been suggested as a potential chemopreventive strategy for nonmelanoma skin cancer.[42-44] In one study, 240 patients with 10–40 AKs received 200 mg of celecoxib or placebo orally twice daily for 9 months.[45] At study termination, there was no significant difference in the incidence of AKs between the two study arms. As a secondary endpoint, the number of new nonmelanoma skin cancers was evaluated at 11 months, which revealed a statistically significant reduction in new cases in the treatment arm (P = .002).[45] While the number of serious adverse events was similar in both groups, the known cardiovascular side effects of celecoxib may preclude its widespread use.
Investigational Treatment Strategies for the Chemoprevention of Nonmelanoma Skin Cancer
Several therapies for chemoprevention of nonmelanoma skin cancer that are in investigational stages have demonstrated preliminary efficacy.
Photodynamic therapy (PDT). Aminolevulinic acid (ALA) and methyl aminolevulinate (MAL) are precursors of the endogenous photosensitizer protoporphyrin IX, which is a natural fluorophore that preferentially accumulates in dysplastic cells.[46] Topical application of ALA or MAL, followed by exposure to specific wavelengths of light, leads to the selective production of reactive oxygen species and cell death in precancerous cells.[46] This photochemical reaction is the basis for PDT. PDT is currently approved for the treatment of AKs and some nonmelanoma skin cancer subtypes, and it has also been suggested for prophylaxis of nonmelanoma skin cancer. In one small study, 45 patients were randomized to field treatment with 20% ALA/PDT on one half of the face and placebo/PDT on the other half.[47] There was a significant delay in the number of new AKs at all follow-up time points for ALA/PDT-treated areas compared with placebo/PDT.[47] Although preventative PDT has been explored in transplant patients, it has not yet been rigorously studied in immunocompetent individuals.
Virally engineered topical enzymes. The link between skin cancer and UV radiation has been correlated at the molecular level by the observation of specific mutational patterns following UV-mediated DNA damage. The formation of cyclobutane pyrimidine dimers (CPD) following UV exposure has mutagenic potential via formation of C–>T and CC–>TT transitions. Pyrimidine dimers are normally repaired by nucleotide excision repair (NER) enzymes. While NER enzymes are the main mechanism of UV-induced DNA repair in humans, other organisms utilize base excision repair (BER) pathways, which are catalyzed by glycosylase enzymes. Since humans lack the specific glycosylase enzyme required for initiation of the BER pathway, some have suggested their exogenous delivery to promote DNA repair of CPDs.
T4 endonuclease V (T4N5), a bacteriophage-specific DNA glycosylase, augments CPD repair and reduces the frequency of UV-induced mutations in cultured mammalian cells.[48] One study has since tested the ability of T4N5 liposome lotion to reduce the rate of new AKs and BCCs in patients with xeroderma pigmentosum (XP), a disease characterized by defective NER enzymes. In this trial, 30 patients were randomly assigned to 4 groups of 6 individuals in which 4 patients in each group received the T4N5 liposomal lotion and 2 received placebo. Following 1 year of T4N5 liposome lotion use, there was a statistically significant reduction of 68% and 30% in the rate of new AKs and BCCs, respectively, when compared with placebo.[49] No significant safety issues were detected in this study; however, the sample size was small and patients were only followed for 18 months.[49] Despite the efficacy of T4N5 liposomal therapy reported in this study, no additional studies have been performed in XP patients or other populations.
Photolyase. Photolyase, a monomeric DNA repair enzyme, is capable of removing UV-induced CPDs and is found in nearly all living organisms with the exception of placental animals, including humans.[50] In one study, photolyase was derived from Anacystis nidulans, a type of algae, and compounded into photolyase-containing liposomes. Following exposure to UVB radiation, photolyase solution was applied to the irradiated skin of 19 healthy individuals. The treated area was covered for 1 hour and then exposed to photoreactivating radiation (340–450 nm, maximum at 365 nm) for 30 minutes. Subsequent molecular analysis demonstrated that topical photolyase followed by photoreactivating radiation was effective in at least partial removal of UVB-induced CPDs.[50]
KEY POINTS
- Implementation of secondary prevention strategies are important for patients at high risk for development of nonmelanoma skin cancers.
- Use of chemopreventive agents such as nicotinamide may be an effective secondary prevention strategy for select patients.
- Additional research is required to evaluate the efficacy of novel chemopreventive agents for the prevention of nonmelanoma skin cancers.
In another study, Anacystis nidulans photolyase was added to traditional sunscreen. The primary endpoint of this study was to determine if the addition of photolyase decreased the formation of CPDs and prevented UV radiation–induced cell apoptosis.[51] In a 6-month-long RCT in patients with AKs, the clinical and molecular effects of sunscreen plus DNA repair enzymes vs traditional sunscreen were compared. They found that addition of photolyase reduced UV-induced DNA damage by 61% when compared with baseline values (P < .001).[52]
Chemoprevention of Nonmelanoma Skin Cancer in Transplant Patients
Nonmelanoma skin cancer management in immunosuppressed patients has significant challenges; chemoprevention in this population has been best studied in the setting of solid organ transplantation.
Chronic immunosuppression following organ transplantation is a well-known risk factor for the development of nonmelanoma skin cancer.[53] Due to the high incidence of nonmelanoma skin cancer in transplant recipients, chemoprophylaxis has been suggested in high-risk patients, including those who develop multiple skin cancers per year or patients who present with clinically aggressive cases.
Though several agents have been proposed, acitretin-a systemic retinoid-has been most rigorously studied. In an open-label randomized crossover trial, 23 renal transplant recipients with a previous history of nonmelanoma skin cancer received 25 mg/d of acitretin (or dose modification based on tolerability-up to 50 mg/d) for 12 months. Though there were significantly fewer SCCs observed when patients were taking acitretin compared with the drug-free period (P = .002), there were no effects on BCCs. Only 11 patients were able to complete the 2-year trial, with treatment side effects necessitating study withdrawal for 9 patients.[54] In another study, 44 renal transplant recipients with ≥ 10 AKs were enrolled in a double-blind placebo-controlled RCT to evaluate the chemoprophylactic effects of 30 mg of acitretin daily. Following the 6-month treatment period, there was a statistically significant difference in the number of new SCCs between the treatment and placebo arms. Three patients in the acitretin arm were removed from study early: 1 for hypercholesterolemia and 2 due to drug rash.[55] In a third study with renal transplant recipients, comparing the effect of 2 different doses of acitretin (0.4 mg/kg/d for 1 year vs 0.4 mg/kg/d for 3 months followed by 0.2 mg/kg/d for the remaining 9 months), no effect on the incidence of new skin malignancies was detected.[56] Importantly, acitretin had no adverse effects on renal function in any of these studies.[54-56]
Given the available evidence, acitretin may be efficacious for chemoprevention of nonmelanoma skin cancer in high-risk transplant patients; however, optimal dosing and long-term safety remain unclear. While other agents such as nicotinamide, capecitabine, and PDT have been proposed for nonmelanoma skin cancer chemoprevention in transplant patients, the evidence to support their use is limited and not recommended at this time.[57-63]
Conclusion
Disease prevention encompasses a range of interventions aimed at early detection of disease, as well as reduction of disease burden once damage has already occurred. There is a clear need for development of evidence-based guidelines for skin cancer screening. In the absence of data-driven guidelines, we recommend that high-risk individuals (see Table 1) undergo regular skin exams by a dermatologist. It is our practice to follow patients with skin examination every 3 to 12 months based upon individual clinical characteristics, including risk factors, rate of new nonmelanoma skin cancer development, and patient preference.
In addition to skin cancer screening, chemoprevention may be an appropriate secondary prevention strategy in some patients. The use of chemopreventive agents should be considered in patients who develop multiple nonmelanoma skin cancers per year and/or aggressive tumors, or those who have rapid development of multiple nonmelanoma skin cancers. We generally favor consideration of nicotinamide in high-risk immunocompetent patients due to the favorable toxicity profile and efficacy. Since various therapies target different pathways associated with the development of skin cancer, there may be synergistic effects of combinatorial therapy, and further work is needed to evaluate novel chemopreventive agents alone or in combination for the secondary prevention of nonmelanoma skin cancers.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. American Cancer Society. Cancer facts & figures 2017. Atlanta: American Cancer Society; 2017.
2. Brougham ND, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106:811-5.
3. Brantsch KD, Meisner C, Schonfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713-20.
4. von Domarus H, Stevens PJ. Metastatic basal cell carcinoma: report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-60.
5. Burdon-Jones D, Thomas P, Baker R. Quality of life issues in nonmetastatic skin cancer. Br J Dermatol. 2010;162:147-51.
6. Adami HO, Day NE, Trichopoulos D, Willett WC. Primary and secondary prevention in the reduction of cancer morbidity and mortality. Eur J Cancer. 2001;37:118-27.
7. Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978-86.
8. Centers for Disease Control and Prevention. What can I do to reduce my risk of skin cancer? http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed March 9, 2018.
9. American Cancer Society. Skin cancer prevention and early detection. https://www.cancer.org/cancer/skin-cancer/prevention-and-early-detection.html. Accessed March 9, 2018.
10. American Academy of Dermatology. Prevent skin cancer. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed March 9, 2018.
11. Kiiski V, de Vries E, Flohil SC, et al. Risk factors for single and multiple basal cell carcinomas. Arch Dermatol. 2010;146:848-55.
12. Qureshi AA, Wei-Passanese EX, Li T, Han J. Host risk factors for the development of multiple non-melanoma skin cancers. J Eur Acad Dermatol Venereol. 2013;27:565-70.
13. Chuang TY, Brashear R. Risk factors of non-melanoma skin cancer in United States veterans patients: a pilot study and review of literature. J Eur Acad Dermatol Venereol. 1999;12:126-32.
14. Iannacone MR, Wang W, Stockwell HG, et al. Patterns and timing of sunlight exposure and risk of basal cell and squamous cell carcinomas of the skin – a case-control study. BMC Cancer. 2012;12:417.
15. English DR, Armstrong BK, Kricker A, et al. Case-control study of sun exposure and squamous cell carcinoma of the skin. Int J Cancer. 1998;77:347-53.
16. Vitasa BC, Taylor HR, Strickland PT, et al. Association of nonmelanoma skin cancer and actinic keratosis with cumulative solar ultraviolet exposure in Maryland watermen. Cancer. 1990;65:2811-7.
17. van Dam RM, Huang Z, Rimm EB, et al. Risk factors for basal cell carcinoma of the skin in men: results from the Health Professionals Follow-Up Study. Am J Epidemiol. 1999;150:459-68.
18. Centers for Disease Control and Prevention (CDC). Sunburn and sun protective behaviors among adults aged 18-29 years-United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2012;61:317-22.
19. Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
20. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:436-47.
21. Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-11.
22. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-8.
23. Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany: documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-34.
24. Grange F, Woronoff AS, Bera R, et al. Efficacy of a general practitioner training campaign for early detection of melanoma in France. Br J Dermatol. 2014;170:123-9.
25. Garmyn M, Ribaya-Mercado JD, Russel RM, et al. Effect of beta-carotene supplementation on the human sunburn reaction. Exp Dermatol. 1995;4:104-11.
26. Werninghaus K, Meydani M, Bhawan J, et al. Evaluation of the photoprotective effect of oral vitamin E supplementation. Arch Dermatol. 1994;130:1257-61.
27. Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-95.
28. Frieling UM, Schaumberg DA, Kupper TS, et al. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch Dermatol. 2000;136:179-84.
29. Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957-63.
30. Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-81.
31. Gutiérrez-Dalmau A, Revuelta I, Ferrer B, et al. Distinct immunohistochemical phenotype of nonmelanoma skin cancers between renal transplant and immunocompetent populations. Transplantation. 2010;90:986-92.
32. Damian DL. Nicotinamide for skin cancer chemoprevention. Australas J Dermatol. 2017;58:174-80.
33. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-26.
34. Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-500.
35. Moon TE, Levine N, Cartmel B, et al. Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects: a randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol Biomarkers Prev. 1997;6:949-56.
36. Tangrea JA, Edwards BK, Taylor PR, et al. Long-term therapy with low-dose isotretinoin for prevention of basal cell carcinoma: a multicenter clinical trial. Isotretinoin-Basal Cell Carcinoma Study Group. J Natl Cancer Inst. 1992;84:328-32.
37. Levine N, Moon TE, Cartmel B, et al. Trial of retinol and isotretinoin in skin cancer prevention: a randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol Biomarkers Prev. 1997;6:957-61.
38. O’Brien TG, Simsiman RC, Boutwell RK. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975;35:1662-70.
39. Verma AK, Lowe NJ, Boutwell RK. Induction of mouse epidermal ornithine decarboxylase activity and DNA synthesis by ultraviolet light. Cancer Res. 1979;39:1035-40.
40. Bailey HH, Kim K, Verma AK, et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of {alpha}-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res (Phila). 2010;3:35-47.
41. Kreul SM, Havighurst T, Kim K, et al. A phase III skin cancer chemoprevention study of DFMO: long-term follow-up of skin cancer events and toxicity. Cancer Prev Res (Phila). 2012;5:1368-74.
42. Fischer SM, Pavone A, Mikulec C, et al. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol Carcinog. 2007;46:363-71.
43. An KP, Athar M, Tang X, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73-80.
44. Pentland AP, Schoggins JW, Scott GA, et al. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939-44.
45. Elmets CA, Viner JL, Pentland AP, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102:1835-44.
46. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275-92.
47. Apalla Z, Sotiriou E, Chovarda E, et al. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization: a randomized placebo-controlled study. Br J Dermatol. 2010;162:171-5.
48. Yarosh D, Bucana C, Cox P, et al. Localization of liposomes containing a DNA repair enzyme in murine skin. J Invest Dermatol. 1994;103:461-8.
49. Yarosh D, Klein J, O’Connor A, et al. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Lancet. 2001;357:926-9.
50. Stege H, Roza L, Vink AA, et al. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc Natl Acad Sci USA. 2000;97:1790-5.
51. Berardesca E, Bertona M, Altabas K, et al. Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: clues to skin cancer prevention. Mol Med Rep. 2012;5:570-4.
52. Carducci M, Pavone PS, De Marco G, et al. Comparative effects of sunscreens alone vs sunscreens plus DNA repair enzymes in patients with actinic keratosis: clinical and molecular findings from a 6-month, randomized, clinical study. J Drugs Dermatol. 2015;14:986-90.
53. Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatol Surg. 2012;38:1622-30.
54. George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-73.
55. Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-8.
56. de Sevaux RG, Smit JV, de Jong EM, et al. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol. 2003;49:407-12.
57. Chen AC, Martin AJ, Dalziell RA, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016;175:1073-5.
58. Drago F, Ciccarese G, Parodi A. Nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2016;374:789-90.
59. Endrizzi B, Ahmed RL, Ray T, et al. Capecitabine to reduce nonmelanoma skin carcinoma burden in solid organ transplant recipients. Dermatol Surg. 2013;39:634-45.
60. Jirakulaporn T, Endrizzi B, Lindgren B, et al. Capecitabine for skin cancer prevention in solid organ transplant recipients. Clin Transplant. 2011;25:541-8.
61. de Graaf YG, Kennedy C, Wolterbeek R, et al. Photodynamic therapy does not prevent cutaneous squamous-cell carcinoma in organ-transplant recipients: results of a randomized controlled trial. J Invest Dermatol. 2006;126:569-74.
62. Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg. 2010;36:652-8.
63. Wulf HC, Pavel S, Stender I, Bakker-Wensveen CA. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-8.