Signaling Pathways in the Relapse of Glioblastoma
Javier Orozco-Mera, MD, FACS, MSc, and colleagues assess the signaling pathways and molecular mechanisms involved in the relapse of glioblastoma.
ABSTRACT
Background: Glioblastoma is the most common primary neoplasm of the central nervous system. Standard treatment includes surgery with maximum safe resection and radiotherapy plus concomitant and adjuvant chemotherapy; however, almost invariably, tumor relapse occurs. We aimed to describe signaling pathways and molecular mechanisms present in tumor relapse of glioblastoma.
Methods: This systematic review followed the PRISMA guidelines. We searched the PubMed, EMBASE and Web of Science databases. We included studies that enrolled patients 15 years or older with a diagnosis of glioblastoma according to Louis criteria and focused on signaling pathways and molecular mechanisms present in tumor relapse of glioblastoma. The outcome of interest was progression-free survival.
Results: We identified 1470 articles; 31 met the inclusion criteria. From each publication, we obtained the associated markers O-6-methylguanine-DNA methyltransferase, isocitrate dehydrogenase, mRNA, epidermal growth factor receptor (EGFR), p53, and others. All publications were evaluated with the Q-Genie checklist tool for quality assessment.
Conclusions: We identified a wide variety of signaling pathways and molecular processes that are involved in glioblastoma relapse. This diversity would explain intra- and intertumor heterogeneity, treatment evasion, and relapse. However, only a few molecular processes have robust evidence for clinical utility.
Oncology (Williston Park). 2023;37(3):107-117.
DOI: 10.46883/2023.25920986
INTRODUCTION
Glioblastoma (GB) is the most common primary neoplasm of the central nervous system, with an overall incidence of 0.59 to 3.69 per 100,000.1 It has a very poor prognosis, with a mean survival of 12 to 15 months from its diagnosis; only 3% to 5% of patients survive after 3 years. Standard treatment, based on the Stupp protocol,2 includes surgery with maximum safe resection and radiotherapy plus concomitant and adjuvant chemotherapy. Despite this, almost invariably, tumor relapse occurs. In GB, unlike in other types of cancer, mortality is not associated with the appearance of metastasis but rather with tumor relapse, given the tumor’s ability to evade treatment and the limitation in many cases for optimal resection of the neoplasm by the eloquence of the affected areas. Understanding the mechanisms used by the tumor to evade treatment is fundamental to finding new therapeutic targets. Our objective was to describe the signaling pathways and molecular mechanisms present in tumor relapse of GB considering the evolutionary processes of cancer described by Hanahan and Weinberg.3
METHODS
The search followed the declaration of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020.4
Inclusion and exclusion criteria
We included observational, case-control, and retrospective or prospective cohort studies that enrolled patients aged 15 years or older with a GB diagnosis according to the Louis criteria of 2007 updated in 2016; the patients must have received frontline treatment with surgery, followed by chemotherapy (Stupp protocol or otherwise, as indicated by the author) and/or radiotherapy, and must subsequently have experienced tumor relapse. The type of intervention analyzed in each article corresponded to which signaling pathways and molecular mechanisms could have been involved in disease progression according to the cancer hallmarks identified by Hanahan and Weinberg.3 Exclusion criteria included those studies that, at the time of evaluation, did not have information on progression-free survival (PFS). The expected outcome was relapse, defined as the reappearance of tumor lesions documented by images and confirmed by histology. PFS was sought as a criterion for this outcome.
Search strategy
On March 3, 2020, a systematic search was performed in the PubMed, EMBASE, and Web of Science databases, from January 2004 through December 2019; the late end date ensured the inclusion of the most relevant literature at the time. Search terms were selected based on the population/exposure/outcome (PEO) framework and combined using Boolean operators (“AND”, “OR”). Filters were used to limit the results to those using human subjects, written in English, and published within the desired time frame. These terms were used in the search strategy: glioblastoma, methyltransferase, tumor suppressor gene, phosphatidylinositol, cell senescence proto-oncogene, neurofibromin, sustaining proliferative signaling, avoiding growth suppression, avoiding immune destruction, tumors promoting inflammation, dysregulation of cellular energy, DNA repair enzymes, regulatory genes of gene expression, tumor suppressor, signal transduction, genomic instability, cell reprogramming, phosphatidylinositol 3-kinases, ERB-1 genes.
Study selection and data extraction
After removing duplicates, titles and abstracts were screened for their relevance to the scope of this review. Two authors, J.O. and F.P., independently assessed the eligibility of retrieved articles. To determine suitability for inclusion in this review, the full text of each potentially relevant article was analyzed for overall content and compliance with the eligibility criteria. The following data were extracted from each eligible article: authors, year of publication, study design, population studied, molecular process, diagnostic method, and main findings. The outcome of interest was PFS or time to progression (TTP). Disagreements were resolved by consensus.
Quality and risk of bias assessment
The quality and risk of bias of the studies were assessed through the Q-Genie tool, developed by the Quality of Genetic Association Studies.5 Q-Genie consists of 11 items. Each item was rated using a 7-point Likert scale (1 = poor; 2-3 = good; 4-5 = very good; 6-7 = excellent). The overall quality of the articles was classified by comparing the scores for each topic. Studies were classified as good, moderate, or poor quality if their scores were greater than 40, between 32 and 40, and less than 32, respectively.
Synthesis methods
HRs were extracted, along with their 95% CIs and P values. For continuous results, we extracted data from means, standard deviations, and the number of participants in each group. For continuous asymmetric data, data from medians, ranges, and P values were extracted from nonparametric tests. All results are presented in tables.
FIGURE. 2020 PRISMA Flow Diagram
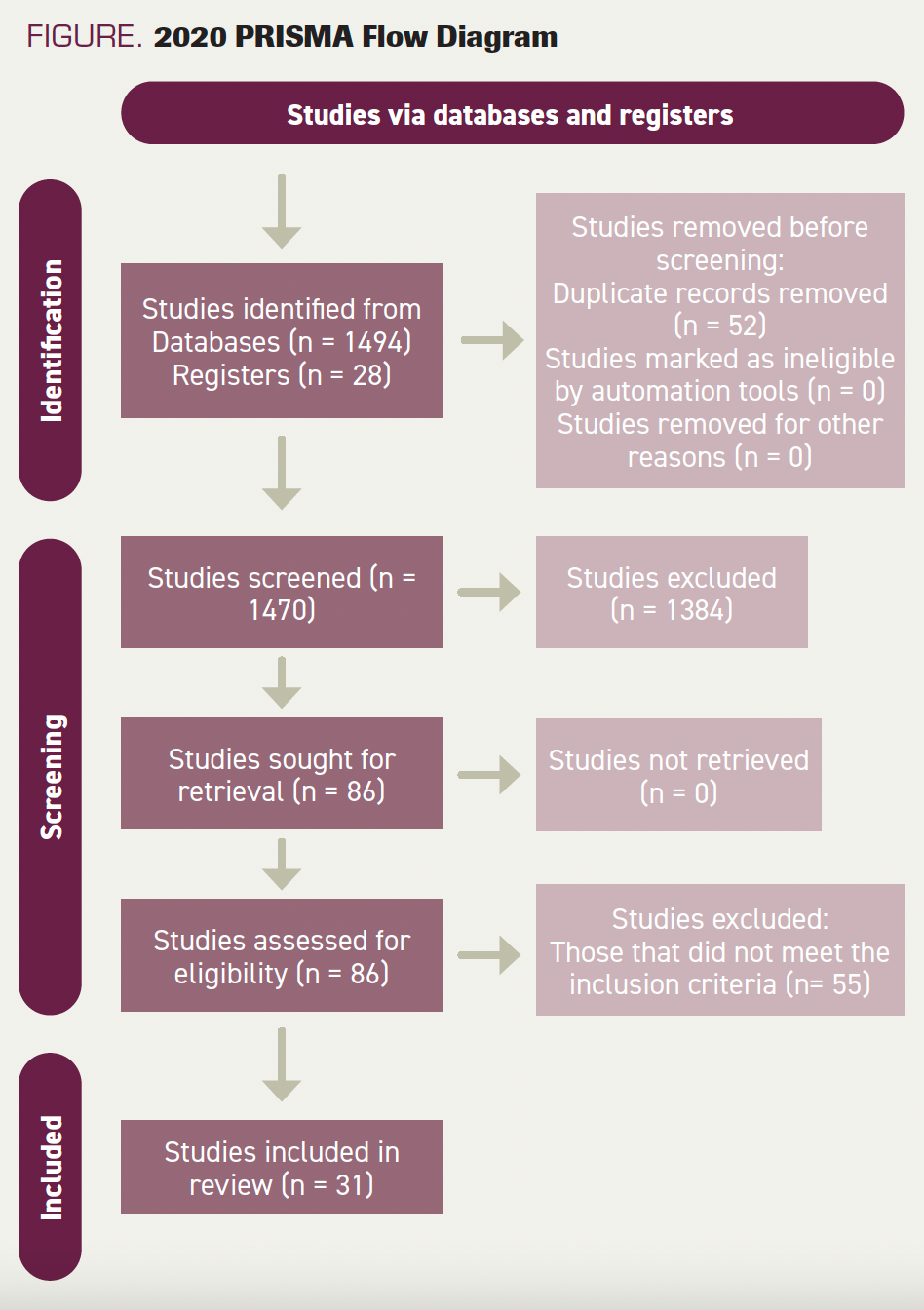
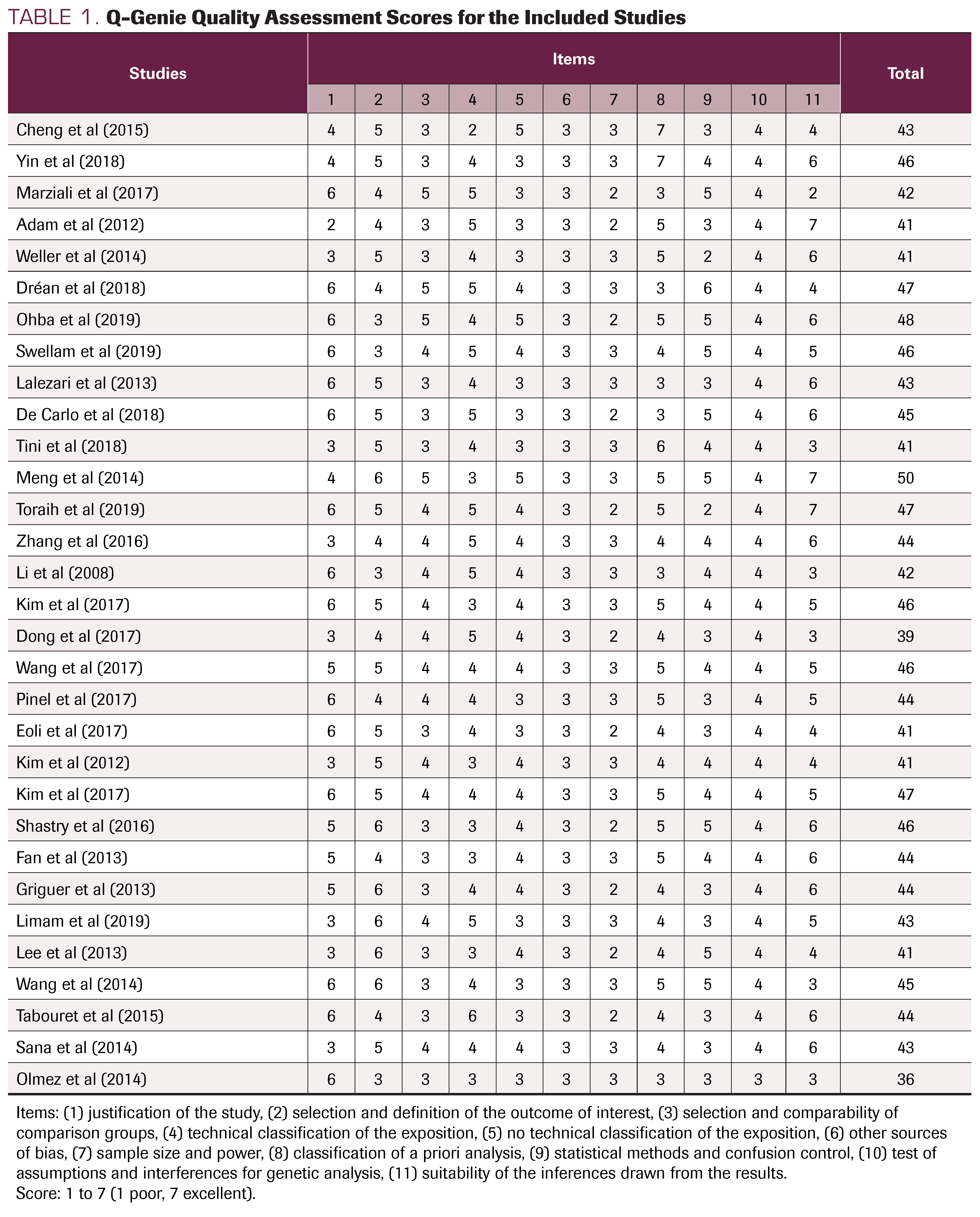
TABLE 1. Q-Genie Quality Assessment Scores for the Included Studies
RESULTS
Study selection
We identified 1470 articles. After the addition of filters, duplication removal, eligibility screening, and final selection, 31 studies were included (Figure).
Quality assessment
A detailed quality rating for each of the 31 articles is shown in Table 1. Two were classified as moderate quality and 29 were classified as good quality; all scored between 36 and 50 on the Q-Genie checklist. In the itemized analysis, we found that most publications have “very good” and “excellent” scores in the following: selection of the working hypothesis, selection and definition of the outcome of interest, a priori planning of the analysis, and ideal inference extracted from the results. The items “statistical methods” and “selection of groups” were generally scored as “good” on the Likert scale (Table 1).
Study and subject characteristics
The study types included were cross-sectional, cohort, and observational follow-up. Table 2 shows all characteristics of included studies. Table 3 presents a detailed description of the signaling pathways and molecular processes that are involved in the relapse of GB. A total of 3585 subjects participated in the 31 studies, with a mean age of 56.05 years (range, 15-90) at the time of GB relapse.
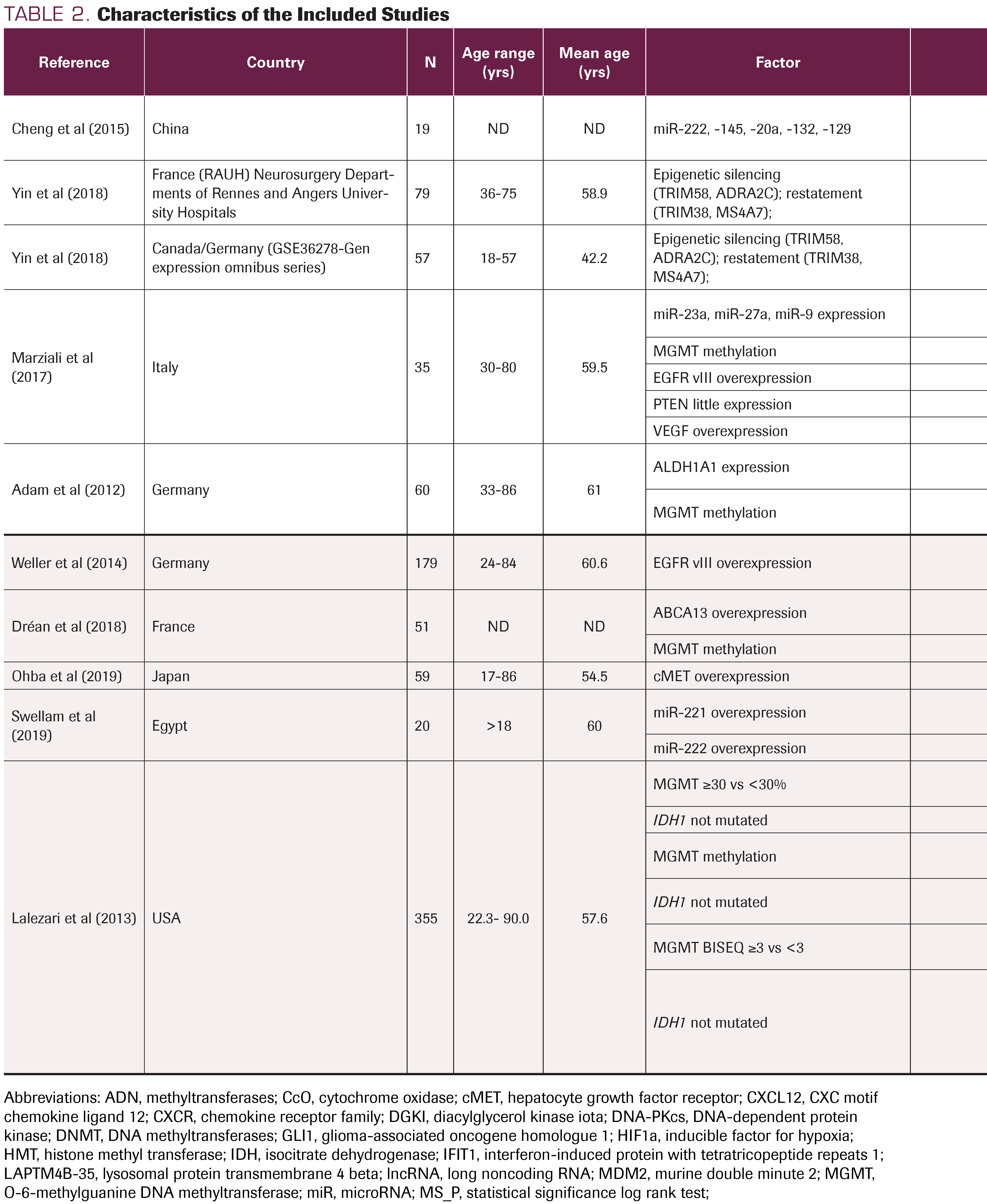
TABLE 2. Characteristics of the Included Studies
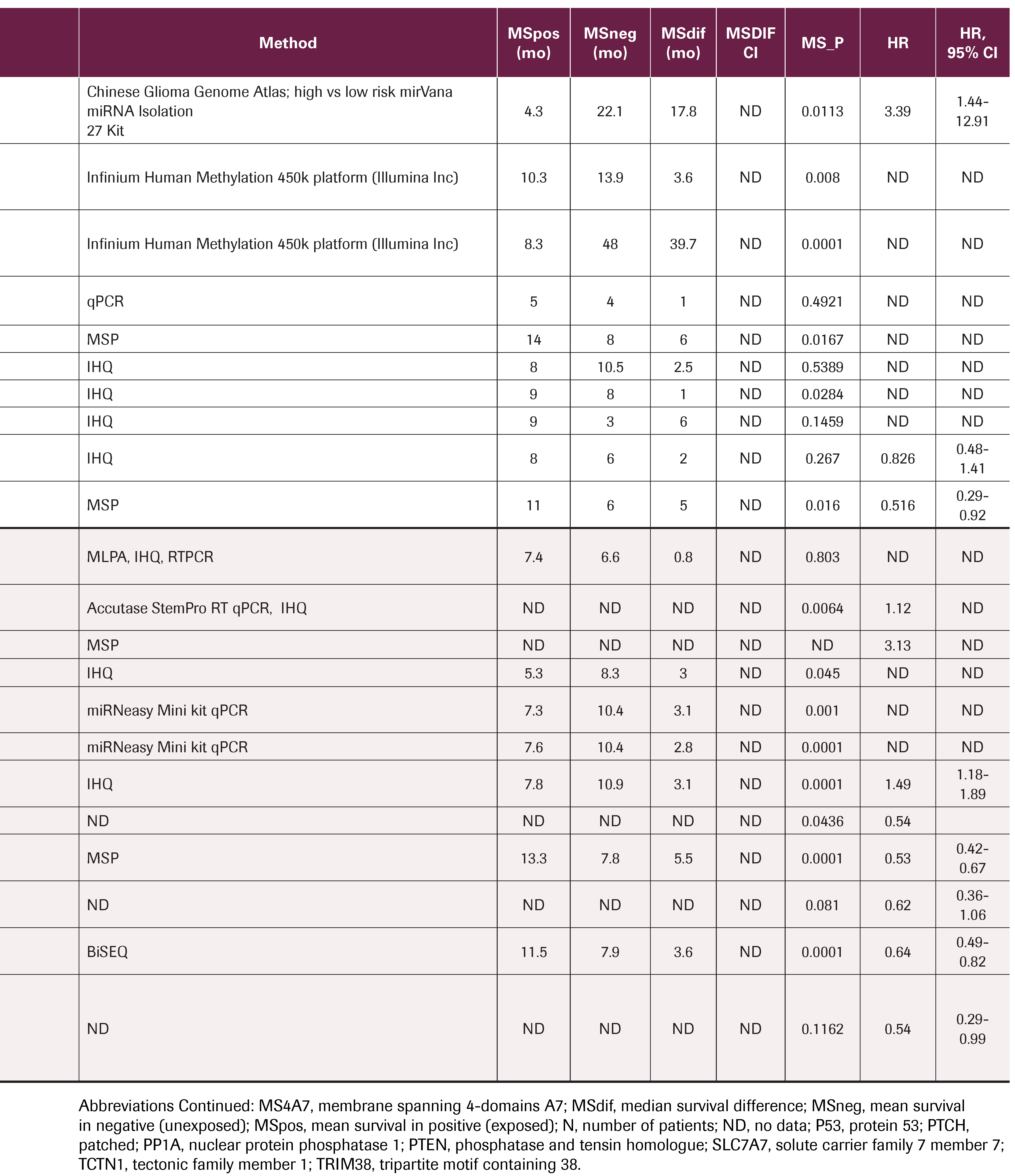
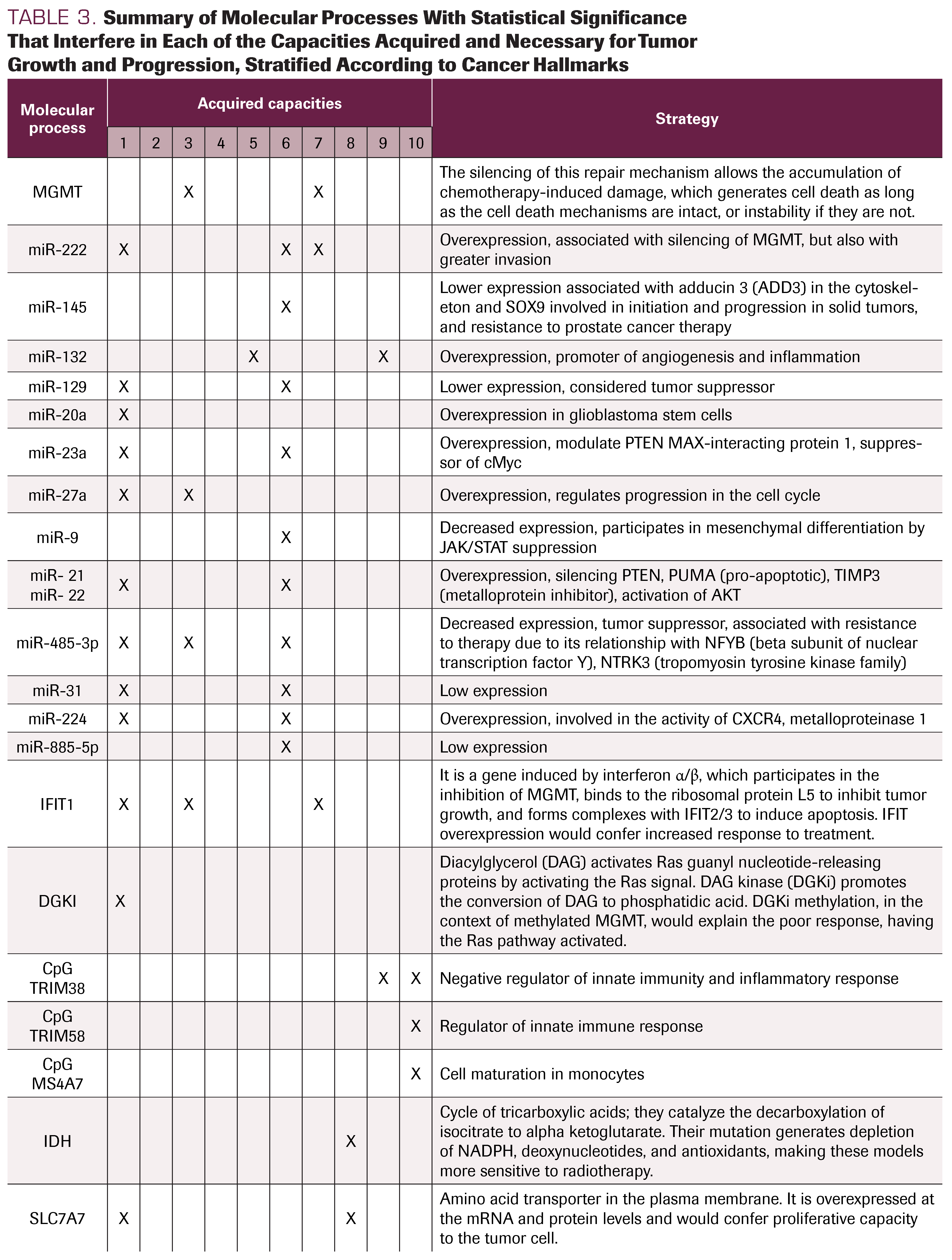
TABLE 3. Summary of Molecular Processes With Statistical Significance That Interfere in Each of the Capacities Acquired and Necessary for Tumor Growth and Progression, Stratified According to Cancer Hallmarks
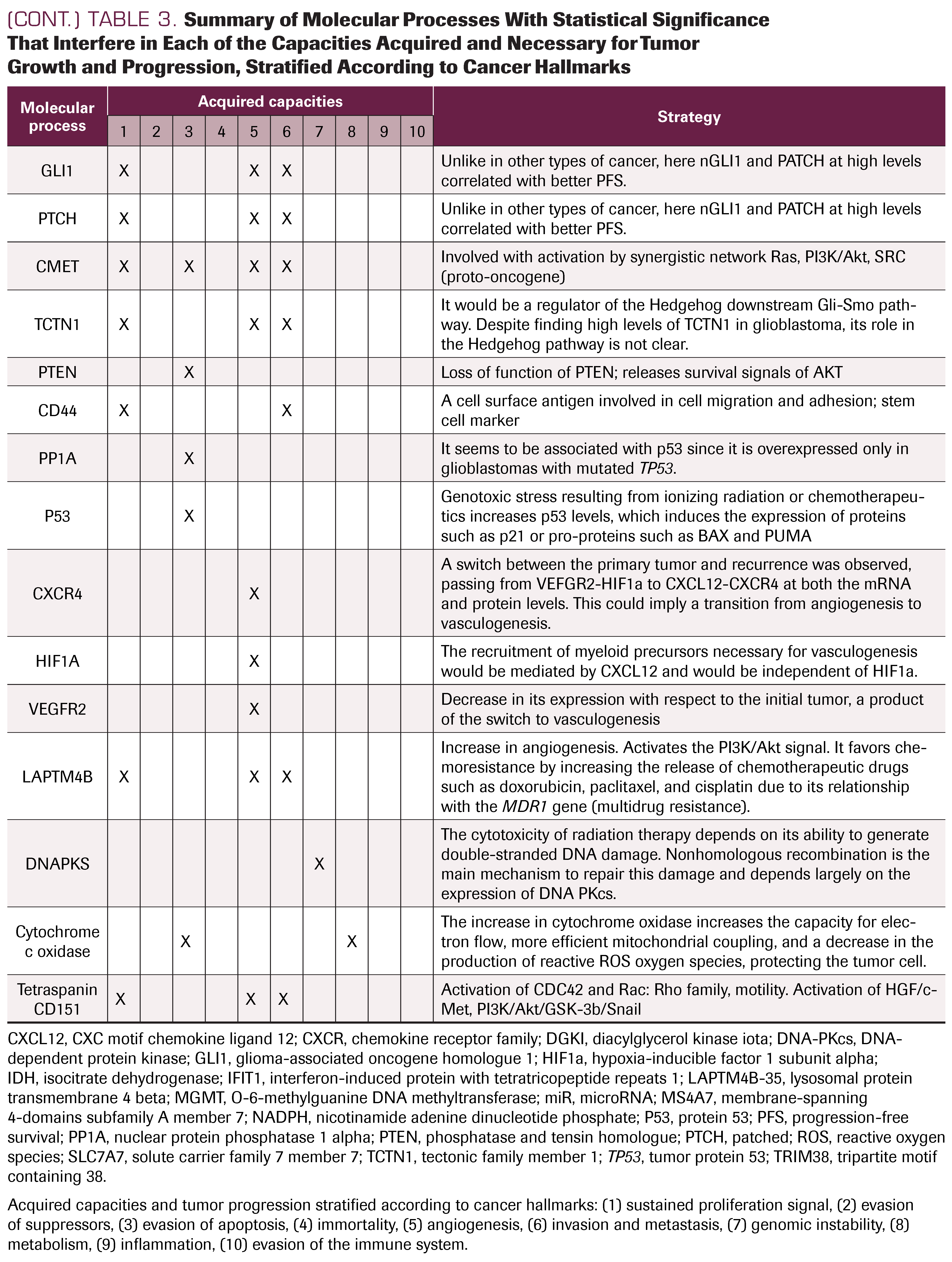
Molecular pathology
The publications describe the processes of deparaffinization, DNA recovery and RNA recovery with different kits, immunohistochemical (IHC) techniques, quantitative polymerase chain reaction (qPCR) with TaqMan and SYBR green probes, multiplex-ligation dependent probe amplification, and high-resolution melting, among other laboratory techniques. The methylation status of O-6-methylguanine DNA methyltransferase (MGMT) was performed in most cases by methylation-specific PCR (MSP) and in others by pyrosequencing (PyroMark Q96 ID). CpG island methylation was measured with Infinium Human Methylation 450k platform (Illumina Inc) and VeraCode GoldenGate Methylation technology (Illumina Inc), among others. The expression of micro-RNA was obtained by mirVana miRNA Isolation 27 Kit and miRNeasy Mini kit; pyrosequencing is the most widely used sequencing technique.
Outcomes
The PFS or TTP was defined by each article as the time between the first surgery and the appearance of a new lesion in imaging studies using McDonald criteria.5 In some previous studies, PFS was measured but not defined, and the outcomes were described as median survival times, which were compared using the log rank test. Some included studies estimated HRs through Cox regression. Quantitative analysis is shown in Table 2.
O-6-methylguanine DNA methyltransferase (MGMT)
MGMT was reviewed in 21 publications. When the method used to identify MGMT methylation status was immunohistochemistry (IHC), Lalezari et al.6, Zhang et al,7 and Wang et al8 reported statistically significant differences (P ≤ .05) in the overall survival of patients with MGMT methylation present, with HRs between 1.49 and 2.162. Limam et al9 did not find statistical significance through this method.
Marziali et al,10 Adam et al,11 Dréan et al,12 Lalezari et al,6 Tini et al,13 Kim et al,14 Eoli et al,15 Kim et al,16 Wang et al,17 Lee et al,18 Limam et al,9 Kim et al,19 and Sana et al.20 measured MGMT status by MSP and reported statistically significant differences (P ≤.05). When the exposure factor was MGMT methylation, they obtained HRs between 0.45 and 0.59; when the exposure factor was unmethylated MGMT, they reported HRs between 1.6 and 2.505.
Limam,9 who did not obtain statistical significance when using IHC, acquired statistically significant differences (HR, 0.096; 95% CI, 0.02-0.46; P = .0001) with MSP. Toraih et al,21 Sadones et al,22 and Pinel et al.23 did not find significant differences.
Isocitrate dehydrogenase
Lalezari et al,6 De Carlo et al,24 Etcheverry et al,25 Wang et al,17 studied the effect of isocitrate dehydrogenase (IDH) with pyrosequencing, finding statistically significant differences for the mutated state, with HRs of 0.12, 0.42, and 0.62, and for the nonmutated state, with an HR of 4.1. Kim19 found no differences using IHC.
Micro-RNA
Cheng et al,26 Swellam et al,27 and Sana et al,20 investigated micro-RNA (miR) signatures, finding statistically significant differences. Cheng et al26 used the mirVana miRNA Isolation 27 Kit, finding overexpression of miR-222, -132, and -129 in the CGGA (Chinese Glioma Genome Atlas), with an HR of 3.39. Swellam et al27 evaluated miR-221 and -222 by qPCR. Sana20 observed that miR-224, miR-432, miR-454, and miR-672 were overexpressed, while miR-31 and miR-885-5p were under expressed (TaqMan Array Human MicroRNA A + B Cards Set v3.0), with an HR of 1.98.
Epidermal growth factor receptor
Tini et al13 measured epidermal growth factor receptor (EGFR) expression by IHC, associating it with statistically significant differences in median survival: P = .003 (HR, 1.79; 95% CI, 1.15-2.8). Eoli et al15 and Limam et al9 did not find statistical significance by the same method. Marziali et al10 and Weller et al28 measured EGFRvIII without finding significant differences.
P53
Li et al29 and Wang et al17 used IHC to evaluate p53 expression or mutations in TP53, finding a significant difference (HR, 0.149) in median survival favoring patients with TP53 mutation. Eoli et al15 and Limam et al9 did not find significant differences. Additionally, Limam et al9 studied MDM2 measured by IHC without finding significant differences.
DISCUSSION
Although the clinical course of each patient with GB is unique and is influenced by the location of the tumor and their age, comorbidities, and Karnofsky Performance Status grade, among other factors, all patients receive the same treatment because no personalized treatment is yet available. Maximum safe resection is performed, in which the tumor is removed macroscopically. The patient subsequently undergoes radiotherapy and concomitant chemotherapy, if their comorbidities allow it, then receives only adjuvant chemotherapy for a generally short time, until the disease appears to be under control. However, relapse occurs in virtually 100% of patients because of the limited treatment options, few of which offer any evidence of increased survival. Cells resistant to multiple therapies persist in the brain parenchyma around the postoperative and postirradiated cavity. Genomic analysis pre- and post-treatment has shown that the recurrent tumor activates pathways associated with clonal and subclonal evolution; these are the origin of treatment failure, development of resistance, and, finally, tumor relapse.
Many molecular pathways and processes are activated during tumor relapse in GB, associated with its evolutionary process. These include: sustained proliferation signals, in which we identified the role of EGFR, miR, IFIT1, DGKI, SLC7A7, GLI1, and PTCH, among others; evasion of apoptosis, with different alterations in TP53, PTEN, PP1A, and MGMT; angiogenesis, associated with the differentiated expression of CXCR4, CXCL12, HIF1α, and VEGFR2; invasion processes, related to miR, tetraspanin CD151, LAPTM4B, and CD44 clusters; genomic instability, associated with alteration in repair mechanisms such as MGMT and DNA PKs (DNA-dependent protein kinase); energy dysregulation or metabolic changes associated with IDH; tumor-promoted inflammation in relation to miR and TRIM38 expression; and, finally, immune system evasion associated with TRIM58 or MS4A7 (Table 3). These processes, already described by Hanahan and Weinberg,3 are not a series of steps accomplished one after another, but rather are launched at different times and in different ways in each tumor, reflecting their intratumoral and intertumoral heterogeneity.
The present review aimed to assess the clinical relevance of the markers identified in each of these different molecular processes or signaling pathways. Some markers have good evidence and clinical applicability, such as MGMT and IDH. Others have substantial theoretical evidence but discrepancies in clinical studies. Some markers that are good candidates emerge rapidly, such as miR and others; these constitute the vast majority of the evidence found, but they require more study in glioblastoma, although they have been described in other types of cancer. Notably, we also found biomarkers with good evidence of their involvement in tumor relapse expressed in terms of PFS, such as MGMT and IDH.
The MGMT gene is located on chromosome 10q26 and encodes a DNA repair protein that removes alkyl groups from the O6-position of guanine. Epigenetic silencing mediated by MGMT promoter methylation generates decreased DNA repair, causing greater chemosensitivity. Moreover, the absence of methylation means that each time an alkylating agent injures the guanine in the O6 position, it is repaired by MGMT, causing chemoresistance.30 This methylation status appears in the multivariate analysis of 21 publications. Whenever a factor associated with recurrence is postulated, it must be verified that it is independent of the possible effect of the methylation status of MGMT. To our knowledge, MGMT methylation confers more chemosensitivity, but it remains uncertain why some tumors with methylation do not respond to initial therapy. Zhang et al11 analyzed IFIT1 and found that MGMT inhibition is associated with its overexpression. Cohen et al,31 when evaluating tumors with MGMT methylation without clinical response, found differential expression in DGKI, a RAS modulator.
IDHs are a group of enzymes fundamental in the tricarboxylic acid cycle. They catalyze the decarboxylation of isocitrate to α-ketoglutarate. IDH1 acts at the level of cytosol and peroxisome, while IDH2/3 acts at the mitochondrial level. IDH1/2 has important functions associated with glucose consumption, lipogenesis, glutathione catabolism, and defense against reactive oxygen species and radiation.32 The IDH1 mutation identified by De Carlo et al24 and Wang et al17 is found more frequently in secondary glioblastomas and generates depletion of NADPH, deoxynucleotides, and antioxidants, making these models more sensitive to radiotherapy and more likely to be associated with a higher PFS. Tumors with nonmutated IDH1, according to Lalezari et al6 and Etcheverry et al,25 are more frequently primary glioblastomas and associated with a lower PFS.
Under normal conditions, in response to genotoxic stress, p53 protein induces cycle stop, mainly in the G1 phase to repair the DNA damage; ultimately, this leads to apoptotic cell death or senescence, thus preventing cell replication with DNA damage. Genotoxic stress secondary to ionizing radiation or chemotherapeutics increases p53 levels, which induces the expression of regulatory cell cycle proteins, such as p21, or pro-apoptotic proteins, such as BAX and PUMA.29 TP53 mutation, studied by Li et al,29 Wang et al,21 Eoli et al,19 and Limam et al,9 is associated with chemosensitivity and higher PFS, while functional p53 is associated with a lower PFS.
Some of the most attractive markers currently under research are miRs: small noncoding RNA molecules, highly conserved, and composed of between 18 and 25 nucleotides. They are responsible for the posttranscriptional negative regulation of gene expression. Bioinformatics tools estimate that miRNAs regulate up to 60% of human genes, including genes associated with chemo- and radioresistance. miRNAs generally act in clusters and adopt the role of oncogenes or tumor suppressor genes, depending on their target. In this way, they can inactivate suppressor genes or activate oncogenes.20
Wang et al,8 when comparing the miRNA profile of glioblastomas with that of healthy subjects in peripheral blood, has found a decrease in the expression of miR-485-3p, suggesting its role as tumor suppressor. Its participation in chemoresistance has also been described. This decrease in expression was associated with lower disease-free survival. Cheng et al26 studied miR 222, which is widely recognized as an oncogene. The miRNAs showed differential expression between tumors with and without MGMT methylation. Therefore, the signature constituted by miR-222, miR-145, miR-20a, miR-132, and miR-129 was postulated by Cheng et al26 as associated with a decrease in PFS even in the presence of promoter methylation.
From our perspective, there is ever-increasing knowledge of gliomagenesis and why successful treatment has been so elusive. We have synthesized a great deal of evidence and framed it in the landmarks of cancer proposed by Hanahan and Weinberg. However, the new therapies have only slightly increased PFS, with no benefit to OS, which shows that we still have “lost pieces.” As such, it is necessary to standardize laboratory tests as well as the exhaustive application of current criteria for the follow-up of patients at the time of evaluating a tumor recurrence. The new therapies have expanded our vocabulary with terms like pseudoprogression and pseudoresponse. We believe that the best way to evaluate these “escape routes” is by analyzing recurrence in terms of the central precepts of molecular biology, and what occurs at many levels: DNA, RNA expression, transcriptional, protein and posttranscriptional, and epigenomic. We have ever-more alternatives framed in immunotherapy, vaccines, target therapies, and bioprospecting studies. Multidisciplinary research is clearly necessary—including basic, clinical, and bioengineering research—which at some point must achieve longer life expectancies for our patients.
Limitations
A limitation of the review is the large amount of isolated data presented, which is useful for research but not as relevant in the clinical field if no other studies exist to support them. Moreover, many studies lack information on PFS, and for this reason 21 studies initially considered were excluded. Information about disease-free survival was requested from all authors via email, but few data were obtained. Finally, the categorization of molecular processes according to the evolutionary processes described is difficult to achieve due to the great integration of the pathways. Occasionally it becomes a somewhat subjective task, although it is very useful to visualize tumor escape pathways.
Another limitation is the lack of standardization in the tests; results may vary according to the proposed analysis. With MGMT, for instance, results differ depending on the technique used. Likewise, there is a lack of standardization for the use of radiological criteria of relapse; some studies still use the McDonald criteria, while others use the RANO or the iRANO criteria.
CONCLUSIONS
It is possible to identify a wide variety of signaling pathways and molecular processes implied in the relapse of GB, as well as in the evolution of many types of cancer. This diversity would explain intra- and intertumor heterogeneity, treatment evasion, and, finally, relapse. However, there are few molecular processes for which robust evidence is available that have resulted in clinical utility. Therefore, it is necessary to subject the candidate processes to additional clinical trials, to build a larger body of evidence that allows personalization of GB therapy. In this context, knowledge of the signaling pathways activated in each case will determine the type of treatment and the temporal pattern of its administration.
AUTHOR AFFILIATIONS:
Javier Orozco-Mera, MD, FACS, MSc1; Fernando Peralta-Pizza, MD2; Oscar Escobar-Vidarte, MD3; Brenda Coll-Tello, MSc4; Aurelio Ariza, MD5
1Department of Neurosurgery, Hospital Universitario del Valle “Evaristo García,” University of Valle; Cali, Colombia; javier.orozco@correounivalle.edu.co
2Department of Neurosurgery, Hospital Universitario del Valle “Evaristo García,” University of Valle; Cali, Colombia; fperaltap@unal.edu.co
3Department of Neurosurgery, HospitalUniversitario del Valle “Evaristo García,” University of Valle; Cali, Colombia; oscar. escobar@correounivalle.edu.co
4Research and Innovation Unit, Hospital Universitario del Valle “Evaristo García,” University of Valle; Cali, Colombia; brenda.coll@correounivalle.edu.co
5Service of Anatomic Pathology, Hospital Germans Trias I Pujol, Autonomous University of Barcelona; Barcelona, Spain; aurelio.ariza@uab.cat
CORRESPONDING AUTHOR:
Javier Orozco- Mera, MD, FACS, MSc
Neurosurgeon
Department of Neurosurgery
Hospital Universitario del Valle “Evaristo García”
University of Valle
Street 5 # 36-08, Valle del Cauca
Cali, Colombia
Phone (+57) 6206000 ext. 1804 / (+57) 5195082
ORCID: 0000-0002-4293-4659
Email: javier.orozco@correounivalle.edu.co
COMPETING INTERESTS: The authors declare they have no conflicts of interest.
FUNDING: This research did not receive external funding.
ACKNOWLEDGMENTS: To Rey Juan Carlos University and Hospital Universitario del Valle “Evaristo García” for the academic space to accomplish this investigation.
ACKNOWLEDGMENTS AND DISCLOSURES: No conflicts of interest are reported by authors. Each author met each of the authorship requirements as stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals, namely the conception, design, acquisition, analysis, interpretation of the data, writing the draft, and final approval.
REFERENCES
- Nguyen HS, Shabani S, Awad AJ, Kausha M, Doan N. Molecular markers of therapy-resistant glioblastoma and potential strategy to combat resistance. Int J Mol Sci. 2018;19(6):1765. doi:10.3390/ijms19061765
- Stupp R, Mason WP, van den Bent MJ, et al; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi:10.1056/NEJMoa043330
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. doi:10.1016/j.cell.2011.02.013
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
- Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13(5):347. doi:10.1007/s11910-013-0347-2
- Lalezari S, Chou AP, Tran A, et al. Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neuro Oncol. 2013;15(3):370-381. doi:10.1093/neuonc/nos308
- Zhang J-F, Chen Y, Lin G-S, et al. High IFIT1 expression predicts improved clinical outcome, and IFIT1 along with MGMT more accurately predicts prognosis in newly diagnosed glioblastoma. Hum Pathol. 2016;52:136-144. doi:10.1016/j.humpath.2016.01.013
- Wang ZQ, Zhang MY, Deng ML, Weng NQ, Wang HY, Wu SX. Low serum level of miR-485-3p predicts poor survival in patients with glioblastoma. PLoS One. 2017;12(9):e0184969. doi:10.1371/journal. pone.0184969
- Limam S, Missaoui N, Abdessayed N, et al. Prognostic significance of MGMT methylation and expression of MGMT, P53, EGFR, MDM2 and PTEN in glioblastoma multiforme. Ann Biol Clin (Paris). 2019;77(3):307-317. doi:10.1684/abc.2019.1448
- Marziali G, Buccarelli M, Giuliani A, et al. A three-microRNA signature identifies two subtypes of glioblastoma patients with different clinical outcomes. Mol Oncol. 2017;11(9):1115-1129. doi:10.1002/1878- 0261.12047
- Adam SA, Schnell O, Pöschl J, et al. ALDH1A1 is a marker of astrocytic differentiation during brain development and correlates with better survival in glioblastoma patients. Brain Pathol. 2012;22(6):788-797. doi:10.1111/j.1750-3639.2012.00592.x
- Dréan A, Rosenberg S, Lejeune F-X, et al. ATP binding cassette (ABC) transporters: expression and clinical value in glioblastoma. J Neurooncol. 2018;138(3):479- 486. doi:10.1007/s11060-018-2819-3. Published correction appears in J Neurooncol. 2018;138(3):487.
- Tini P, Nardone V, Pastina P, et al. Epidermal growth factor receptor expression predicts time and patterns of recurrence in patients with glioblastoma after radiotherapy and temozolomide. World Neurosurg. 2018;109:e662-e668. doi:10.1016/j.wneu.2017.10.052
- Kim J, Lee S-H, Jang JH, Kim M-S, Lee EH, Kim YZ. Increased expression of the histone H3 lysine 4 methyltransferase MLL4 and the histone H3 lysine 27 demethylase UTX prolonging the overall survival of patients with glioblastoma and a methylated MGMT promoter. J Neurosurg. 2017;126(5):1461-1471. doi:10.3171/2016.4.JNS1652
- Eoli M, Menghi F, Bruzzone MG, et al. Methylation of O6-methylguanine DNA methyltransferase and loss of heterozygosity on 19q and/or 17p are overlapping features of secondary glioblastomas with prolonged survival. Clin Cancer Res. 2007;13(9):2606-2613. doi:10.1158/1078-0432.CCR-06-2184
- Kim YS, Kim SH, Cho J, et al. MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys. 2012;84(3):661-667. doi:10.1016/j.ijrobp.2011.12.086
- Wang K, Wang Y-Y, Ma J, et al. Prognostic value of MGMT promoter methylation and TP53 mutation in glioblastomas depends on IDH1 mutation. Asian Pac J Cancer Prev. 2014;15(24):10893-10898. doi:10.7314/ APJCP.2014.15.24.10893
- Lee D, Suh Y-L, Park T-I, et al. Prognostic significance of tetraspanin CD151 in newly diagnosed glioblastomas. J Surg Oncol. 2013;107(6):646-652. doi:10.1002/ jso.23249
- Kim Y, Do I-G, Hong M, Suh Y-L. Negative prognostic effect of low nuclear GLI1 expression in glioblastomas. J Neurooncol. 2017;133(1):69-76. doi:10.1007/s11060- 017-2426-8
- Sana J, Radova L, Lakomy R, et al. Risk score based on microRNA expression signature is independent prognostic classifier of glioblastoma patients. Carcinogenesis. 2014;35(12):2756-2762. doi:10.1093/ carcin/bgu212
- Toraih EA, El-Wazir A, Hussein MH, et al. Expression of long intergenic non-coding RNA, regulator of reprogramming, and its prognostic value in patients with glioblastoma. Int J Biol Markers. 2019;34(1):69-79. doi:10.1177/1724600818814459
- Sadones J, Michotte A, Veld P, et al. MGMT promoter hypermethylation correlates with a survival benefit from temozolomide in patients with recurrent anaplastic astrocytoma but not glioblastoma. Eur J Cancer. 2009;45(1):146-153. doi:10.1016/j.ejca.2008.09.002
- Pinel B, Duchesne M, Godet J, et al. Mesenchymal subtype of glioblastomas with high DNA-PKcs expression is associated with better response to radiotherapy and temozolomide. J Neurooncol. 2017;132(2):287-294. doi:10.1007/s11060-016-2367-7
- De Carlo E, Gerratana L, De Maglio G, et al. Defining a prognostic score based on O6-methylguanine-DNA methyltransferase cut-off methylation level determined by pyrosequencing in patients with glioblastoma multiforme. J Neurooncol. 2018;140(3):559-568. doi:10.1007/ s11060-018-2981-7
- Etcheverry A, Aubry M, Idbaih A, et al. DGKI methylation status modulates the prognostic value of MGMT in glioblastoma patients treated with combined radio-chemotherapy with temozolomide. PLoS One. 2014;9(9):e104455. doi:10.1371/journal.pone.0104455
- Cheng W, Ren X, Cai J, et al. A five-miRNA signature with prognostic and predictive value for MGMT promoter-methylated glioblastoma patients. Oncotarget. 2015;6(30): 29285-29295. doi:10.18632/ oncotarget.4978
- Swellam M, Ezz El Arab L, Al-Posttany AS, Said SB. Clinical impact of circulating oncogenic MiRNA-221 and MiRNA-222 in glioblastoma multiform. J Neurooncol. 2019;144(3):545-551. doi:10.1007/s11060-019-03256- 2
- Weller M, Kaulich K, Hentschel B, et al; German Glioma Network. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int J Cancer. 2014;134(10):2437-2447. doi:10.1002/ ijc.28576
- Li S, Jiang T, Li G, Wang Z. Impact of p53 status to response of temozolomide in low MGMT expression glioblastomas: preliminary results. Neurol Res. 2008;30(6):567-570. doi:10.1179/174313208X297913
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003. doi:10.1056/ NEJMoa043331
- Olmez OF, Cubukcu E, Evrensel T, et al. The immunohistochemical expression of c-Met is an independent predictor of survival in patients with glioblastoma multiforme. Clin Transl Oncol. 2014;16(2):173-177. doi:10.1007/s12094-013-1059-4
- Cohen A, Holmen S, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 2013;13(5):345. doi:10.1007/s11910-013-0345-4