Simultaneous and Sequential Doxorubicin/Docetaxel Equally Safe, Effective in Metastatic Breast Cancer
MEMPHIS-A dose-intense sequential schedule of doxorubicin/docetaxel (Taxotere) plus growth factor was no more effective than standard simultaneous administration in treating metastatic breast cancer.
MEMPHISA dose-intense sequential schedule of doxorubicin/docetaxel (Taxotere) plus growth factor was no more effective than standard simultaneous administration in treating metastatic breast cancer.
Lee Schwartzberg, MD, of Response Oncology, Inc., Memphis, and his colleagues compared the dose-intense sequential schedulegiving maximum tolerated doses of each drug in sequence and shortening the interval between doseswith standard administration of the drugs among 98 randomized patients. Dr. Schwartzberg presented the results at the ASCO annual meeting.
Sequential schedules are attractive because they enable clinicians to deliver full doses of each drug. Dr. Schwartzbergs group assigned patients with metastatic breast cancer who had received no prior chemotherapy for metastatic disease to receive an equal total dose of doxorubicin and docetaxel as induction treatment.
Patients were randomized to either simultaneous administration (doxorubicin 45 mg/m² + docetaxel 75 mg/m² every 21 days for four cycles) or sequential administration (doxorubicin 90 mg/m² every 14 days for two cycles followed by docetaxel 100 mg/m² every 14 days for three cycles).
G-CSF (Neupogen) was scheduled on days 3 to 10 in the sequential arm but given only after a neutropenic episode in the simultaneous arm.
Response was assessed after induction. Responding patients were eligible for high-dose consolidation.
Dose Densities Differ
Dr. Schwartzberg reported that 37% of the patients had metastatic disease at diagnosis, 54% had prior adjuvant therapy, and 31% had liver involvement. Median delivered dose intensity and cumulative dose for both drugs were similar in both arms.
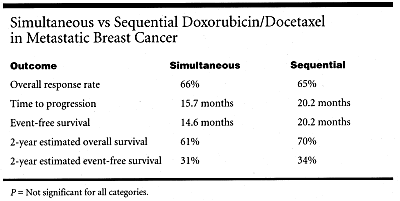
The median dose density was markedly higher for the sequential arm, but this did not translate into greater efficacy. Although researchers were able to deliver more than 90% of the planned doses, the overall response rate, time to progression, event-free survival, and overall survival were about the same on both regimens, according to Dr. Schwartzberg (see Table).
There was significantly more neutropenia in the simultaneous arm of the study, but more skin toxicity (a painful erythroderma beginning in the hands and feet and extending to the total body) in the sequential arm.
Delivered dose density is higher with the sequential schedule, but without an improvement in response rate, event-free survival, or overall survival, Dr. Schwartzberg concluded. A dose-dense, growth-factorsupported sequential treatment offers no advantage over a standard combination schedule.
Quality-of-Life Concerns
In discussing this study, Clifford Hudis, MD, of Memorial Sloan-Kettering Cancer Center, said, Sequential vs concurrent doxorubicin/docetaxel had no difference in efficacy and little difference in toxicity. This was a disappointing result...but not a completely fair test of dose-dense chemotherapy.