Small-cell lung cancer,mesothelioma, and thymoma
As discussed in chapter 6, there are two major subdivisions of lung cancer:small-cell lung cancer (SCLC), for which chemotherapy is the primary treatment,and non-small-cell lung cancer (NSCLC). SCLC is decreasing in frequencyin the United States, with recent data showing it represents only 14%of lung cancers. This chapter provides information on the staging and prognosis,pathology and pathophysiology, treatment, and follow-up of longtermsurvivors of SCLC and concludes with brief discussions on mesotheliomaand thymoma.
As discussed in chapter 6, there are two major subdivisions of lung cancer:small-cell lung cancer (SCLC), for which chemotherapy is the primary treatment,and non-small-cell lung cancer (NSCLC). SCLC is decreasing in frequencyin the United States, with recent data showing it represents only 14%of lung cancers. This chapter provides information on the staging and prognosis,pathology and pathophysiology, treatment, and follow-up of longtermsurvivors of SCLC and concludes with brief discussions on mesotheliomaand thymoma.Chapter 6 provides information on the epidemiology, etiology, screeningand prevention, and diagnosis of lung cancer in general and covers NSCLCand carcinoid tumors of the lungs.SMALL-CELL LUNG CANCERStaging and prognosisThe TNM staging system, used for all NSCLC patients, does not predict wellfor survival in SCLC patients and is generally not utilized in SCLC, exceptfor surgical staging (see chapter 6, Table 1). Rather, SCLC is usually describedas either limited (M0) or extensive (M1), although these general termsare inadequate when evaluating the role of surgery. Patients with SCLC whohave stages I-III disease, excluding those with a malignant pleural effusion,are classified as having limited disease. These patients constitute approximatelyone-third of all SCLC patients. The remaining SCLC patients fall intothe extensive-disease category, which includes any patient with a malignantpleural effusion or any site of distant disease-brain, liver, adrenal gland,bone, bone marrow, and others.The staging of lung cancer must be conducted in a methodical and detailedmanner to permit appropriate therapeutic recommendations and to allowcomparison of treatment results from different institutions.Stage is commonly reported as either clinical or pathologic. The former isbased on noninvasive (or minimally invasive) tests, whereas the latter is basedon tissue obtained during surgery (see chapter 6).The most important prognostic factor in lung cancer is the stage of disease.Within a given disease stage, the next most important prognostic factors areperformance status and recent weight loss. The two scales used to defineperformance status are the Eastern Cooperative Oncology Group (ECOG)performance status system and the Karnofsky system (see Appendix 1). Inshort, patients who are ambulatory have a significantly longer survival. Thosewho have lost ≥ 5% of body weight during the preceding 3-6 months have aworse prognosis.Pathology and pathophysiologySCLC tends to present with a large central lung mass and associated extensivehilar and mediastinal lymphadenopathy. Clinically evident distant metastasesare present in approximately two-thirds of patients at diagnosis.Additionally, data from autopsy examination indicate micrometastatic diseasein 63% of patients who died within 30 days of attempted curative resectionof SCLC. Thus, it is a systemic disease at presentation in the majority ofpatients.SCLC is a small, blue, round cell tumor that is primitive and undifferentiatedat the light microscopic level. Electron microscopy demonstrates its neuroendocrinederivation by the presence of dense core granules. The immunohistochemicalevidence of neuroendocrine derivation includes positive stainingfor chromogranin, synaptophysin, and other proteins. The APUD (amineprecursor uptake and decarboxylation) machinery present in the dense coregranule leads to the production of biologically active amines and promotesthe synthesis of polypeptide hormones such as ADH and ACTH. Paraneoplasticsyndromes due to hormone excess result. The most common ofthese syndromes, syndrome of inappropriate antidiuretic hormone secretion(SIADH), occurs in approximately 10% of patients with SCLC.Hypercortisolism and a Cushing's-like syndrome are more rare, seen in only1%-2% of patients.TreatmentTREATMENT OF DISEASE LIMITED TO LUNG PARENCHYMASurgery
The majority of patients with SCLC present with advanced-stage disease. Inthe 5%-10% of patients whose tumor is limited to the lung parenchyma, very often the diagnosis is established only after the lung mass has been removed.If, however, the histology has been determined by bronchoscopic biopsy orfine-needle aspiration and there is no evidence of metastatic disease followingextensive scanning, examination of the bone marrow, and biopsy of themediastinal lymph nodes, resection should be performed. Adjuvant chemotherapyis recommended because of the high likelihood of the developmentof distant metastases following surgery.The surgical approach in SCLC is similar to that used in NSCLC: A lobectomyor pneumonectomy should be followed by a thorough mediastinallymph node dissection. Tumor resection in SCLC should be limited to patientswho have no evidence of mediastinal or supraclavicular lymph nodemetastases. Recent data suggest that patients with SCLC, presenting as asolitary pulmonary nodule and proven pathologically to be stage I, have a5-year survival rate of ~70% when treated with resection and adjuvantchemotherapy.TREATMENT OF DISEASE LIMITED TO THE THORAX
Approximately one-third of SCLC patients present with disease that is limitedto the thorax and can be encompassed within a tolerable radiation portal.In early studies in which either radiation therapy or surgery alone wasused to treat such patients, median survival was only 3-4 months, and the 5-year survival rate was in the range of 1%-2%. The reason for the failure ofthese therapies was both rapid recurrence of intrathoracic tumor and developmentof distant metastasis.Chemotherapy
During the 1970s, it became apparent that SCLC was relatively sensitive tochemotherapy. Various combination chemotherapy regimens were used totreat limited SCLC. Although none of the regimens was clearly superior,median survival was approximately 12 months, and the 2-year survival ratewas approximately 10%-15%.It appears that maintenance chemotherapy adds little to survival in patientswith limited SCLC.CHEMOTHERAPY PLUS THORACIC IRRADIATION
One of the major advances in treating SCLC in the past 15 years is therecognition of the value of early and concurrent thoracic chemoradiationtherapy. This advance was clearly facilitated by the increase in therapeuticindex when PE (cisplatin [Platinol]/etoposide) chemotherapy is given withthoracic irradiation, as opposed to older anthracycline or alkylator-basedregimens. Although the major impact from this approach is improvedlocoregional control, there are also hints from randomized trials that earlycontrol of disease in the chest can also reduce the risk of distant metastasis.An intergroup trial directly compared once-daily with twice-daily fractionation(45 Gy/25 fractions/5 weeks vs 45 Gy/30 fractions/3 weeks) given at thebeginning of concurrent chemoradiation therapy with PE. Initial analysis showed excellent overall results, with mediansurvival for all patients of 20 monthsand a 40% survival rate to 2 years. With aminimum follow-up of 5 years, survival wassignificantly better in the twice-daily than inthe once-daily irradiation group (26% vs16%). The only difference in toxicity was atemporary increase in grade 3 esophagitis inpatients receiving twice-daily radiationtherapy.Outcomes for patients with limited-stageSCLC have improved significantly over thepast 20 years. In an analysis of phase IIItrials during this time period, median survivalwas 12 months in the control arm in 26phase III studies initiated between 1972 and1981, compared with 17 months in studiesbetween 1982 and 1992 (P < .001). Five studies demonstrated a statisticallysignificant improvement in survival in the experimental arm compared withthe control arm. Interestingly, all five studies involved some aspect of thoracicradiation therapy (three trials compared chemotherapy alone vschemoradiation therapy; one compared early with late radiation therapy;and one compared daily vs twice-daily thoracic radiation therapy). Similarly,data from the Surveillance, Epidemiology, and End Results (SEER) databasedemonstrate that the 5-year survival rate has more than doubled from1973 to 1996 (5.2% vs 12.2%, P = .0001).Current recommendations Although important questions remain as to theoptimal radiation doses, volumes, and timing with regard to chemotherapy,a reasonable present standard is to deliver thoracic irradiation concurrentlywith PE chemotherapy (cisplatin [60 mg/m2 IV on day 1] and etoposide[120 mg/ m2 IV on days 1-3]). An attempt is made to integrate thoracic irradiationduring cycle 1 or 2. Hyperfractionated accelerated fractionation shouldbe considered, given the results of the intergroup 0096 trial. The data extantdo not indicate that chemotherapy beyond four cycles has a favorable impacton long-term outcome.Irradiation can be incorporated sequentially to chemotherapy; however, thisapproach appears to be inferior to early concurrent therapy and should bereserved for use in those for whom concurrent approaches are predicted tobe excessively toxic.Results of an intergroup trial indicate that radiation therapy strategies thatincrease biologic dose can improve local control and survival. Further explorationof accelerated fractionation or conventional doses > 45 Gy is warrantedand is currently being investigated in prospective trials.Movsas et al reported the results of the first Patterns of Care Study (PCS) forlung cancer in the United States. This study was conducted to determine the national patterns of radiotherapy practice inpatients treated for nonmetastatic lung cancerin 1998-1999. As supported by clinicaltrials, patients with limited-stage SCLC receivedchemotherapy plus radiotherapy moreoften than radiotherapy alone (92% vs 5%,P < .0001). However, the median radiotherapydose was 50 Gy, 80% at 1.8-2.0 Gyper fraction. Only 6% of patients receivedhyperfractionated (twice-daily) radiotherapy.A total of 22% received prophylactic cranialirradiation (PCI), with a median dose of30 Gy in 15 fractions. As key studies supportingtwice-daily radiotherapy in PCI andNSCLC were published in 1999, the penetrationof these trials will be assessed in thenext PCS lung survey.Interestingly, Choi et al reported long-termsurvival data from their phase I trial assessingchemotherapy with either standard dailyradiotherapy or accelerated twice-daily radiotherapyas from the Cancer and LeukemiaGroup B (CALGB) 8837 trial. They previouslyreported that the maximum tolerateddose was 45 Gy in 30 fractions for twice-daily radiotherapy and > 70 Gyin 35 fractions for once-daily radiotherapy. The 5-year survival estimated(from this phase I trial) for the twice-daily arm was 20%, vs 36% for the oncedailyradiotherapy arm. They suggest a phase III randomized trial to comparestandard daily radiotherapy (to 70 Gy) vs twice-daily radiotherapy (to45 Gy). Indeed, the long-term results of a phase III trial comparing oncedailyirradiation (to 50.4 Gy in 28 fractions) vs twice-daily irradiation (to 48Gy in 32 fractions via a split course) demonstrated similar outcomes in eitherarm. The median and 5-year survival rates of patients in this study (21 monthsand 20%, respectively) were similar to those reported by Turrisi et al.Although surgical resection is not part of the standard therapy for SCLC, theJapanese Clinical Oncology Lung Cancer Study Group reported the resultsof a phase II trial of postoperative adjuvant cisplatin/etoposide in patientswith completely resected stages I-IIIA SCLC. The 5-year survival rates (in acohort of 62 patients) for pathologic stages I, II, and IIIA SCLC were 69%,38%, and 40%, respectively.Prophylactic cranial irradiation
Recognition that patients with SCLC were at high risk for the developmentof brain metastases led to the suggestion that they be given PCI to preventthe clinical manifestation of previously present but occult CNS disease. Therole of PCI has been controversial. Most trials have shown a reduction in CNS relapse rates but little effect on survival.There also has been concern about thecontribution of PCI to the late neurologicdeterioration seen in some patients withSCLC, although recent studies show neurologicimpairment in many patients with SCLCprior to any treatment.A meta-analysis of all randomized trials ofPCI in patients with SCLC who achieved acomplete or near-complete response to inductionchemotherapy (alone or combinedwith thoracic irradiation) showed a statisticallysignificant improvement in survival inpatients treated with PCI (20.7% at 3 yearsvs 15.3% in those not given PCI). The survivalimprovement with PCI was seen in allpatient subgroups, regardless of age, stageof disease, type of induction treatment, orperformance status.Model calculations from data on patterns offailure in patients achieving a systemic completeresponse suggest that the greatest gainin survival to be expected with PCI is in therange of 5%. To demonstrate this convincinglywould require randomized trials ofabout 700 patients-substantially larger thantrials conducted to date. However, the recentmeta-analysis of randomized trials ofPCI in SCLC patients achieving completeor near-complete response of systemic diseaseshowed a survival improvement of thismagnitude with PCI.Current recommendations When PCI isto be used, patients should be treated onlyif they have achieved a complete or nearcompleteremission of disease outside the CNS. Use of chemotherapeuticagents with known CNS toxicity (eg, methotrexate, procarbazine [Matulane],and nitrosoureas) should be avoided, and chemotherapy should not be givenduring or after irradiation.Radiation doses for PCI should probably be in the range of 25-30 Gy, with adaily fraction size of 2.0-2.5 Gy, although recent data suggest that such dosesdelay and reduce rates of CNS relapse but may not eliminate it; thus, higherdoses may warrant exploration. Larger fraction sizes would be expected toproduce greater toxicity. Smaller fractions given twice daily may reduce toxicity,and trials of this approach are under way.
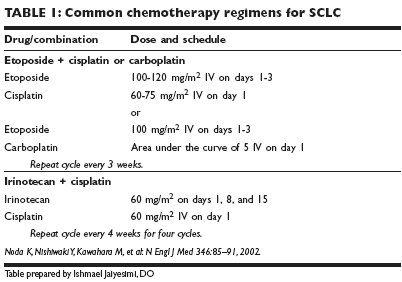
TREATMENT OF EXTENSIVE DISEASE
As mentioned previously, two-thirds of SCLC patients have extensive diseaseat diagnosis. Without treatment, median survival in this group of patientsis 6-8 weeks. Treatment with combination chemotherapy increases themedian survival duration to approximately 8-10 months.Combination chemotherapy
The combination of cisplatin or carboplatin (Paraplatin)/etoposide (see Table1 for common dose ranges) is considered the standard of care in the UnitedStates at this time. This is primarily based on therapeutic index, as randomizedtrials have not demonstrated a survival benefit for this combinationrelative to the older regimen of cyclophosphamide (Cytoxan, Neosar), doxorubicin,and vincristine. The regimen is repeated at 3-week intervals for fourto six courses. Randomized trials of maintenance chemotherapy, either withother drugs or reduced doses of the induction regimen, show improvementin the duration of remission but no impact on overall survival.New agents
A variety of novel agents have been investigated in SCLC. Of these agents,the taxanes and topoisomerase I inhibitors, particularly topotecan (Hycamtin),have demonstrated the greatest efficacy.Taxanes Because of their novel mechanism of action and clinical activity inother solid tumors, including NSCLC, the taxanes-paclitaxel and docetaxel(Taxotere)-are particularly attractive agents for evaluation in the treatmentof SCLC.Paclitaxel Ettinger et al reported that single-agent paclitaxel produces anoverall response rate of 34% in untreated patients with SCLC. This taxane iscurrently being evaluated in combination with a variety of different agents,including etoposide and cisplatin (or carboplatin), in SCLC patients.Docetaxel Compared with paclitaxel, docetaxel appears to have a slightly lowerresponse rate of 26% (12 of 46 patients), even when administered at a dose of100 mg/m2, as reported by the Southwest Oncology Group (SWOG). Thislower response rate with docetaxel was offset somewhat by the fact thatmedian survival was promising at 9 months, similar to that obtained withcombination chemotherapy. Disturbingly, however, median time to diseaseprogression was only 3 months.Topoisomerase I inhibitors The topoisomerase I inhibitors-topotecan andirinotecan (CPT-11 [Camptosar])-are clearly active in SCLC, with singleagen-response rates of 40%-60%. Due to their novel mechanism of action,the topoisomerase I inhibitors have been studied in patients with recurrentand refractory SCLC, as many of these patients have been previously exposedto topoisomerase II inhibitors (epipodophyllotoxins/anthracyclines)during the induction phase of therapy. Furthermore, preclinical data suggestthat topoisomerase I levels are upregulated in cells resistant to topoisomeraseII inhibitors, via downregulation of topoisomerase II levels.Topotecan A randomized, phase III trial compared topotecan vs CAV (cyclophosphamide,doxorubicin [Adriamycin], vincristine) in patients who had aresponse to initial therapy and a minimum drug-free interval of 60 days.Overall response rates were 24% for topotecan alone vs 18% for CAV(P > .05). Time to disease progression and median survival also were similarin the two arms. However, topotecan offered superior palliation for manydisease-related symptoms, including dyspnea, fatigue, and hoarseness, andalso improved patient's ability to carry out daily tasks. Moreover, topotecanhad toxicities similar to those of CAV, with the exception of a slight increasein grade 4 thrombocytopenia.Irinotecan also has been investigated in recurrent or refractory SCLC in alimited number of patients. Like topotecan and other new agents, irinotecanproduced a disappointingly low response rate among patients with SCLCresistant to primary chemotherapy, with only 1 of 27 patients exhibiting aresponse. In contrast, the response rate to irinotecan among patients withinitially sensitive disease that later recurred was 29%.Data from Japan suggest a 4-month overall survival benefit for irinotecanand cisplatin combined as induction therapy for patients with extensive disease.The data await confirmation; a randomized American trial is ongoing.Other agents, such as gemcitabine (Gemzar) and vinorelbine (Navelbine),have shown activity in SCLC, but it has been less impressive than that reportedfor the taxanes and topoisomerase I inhibitors.Phase II trials of new combinations, such as PET (cisplatin [Platinol]/etoposide/paclitaxel [Taxol]) and topotecan/paclitaxel, have yielded promisingmedian and 2-year survival estimates in patients with extensive disease.Three phase III trials testing PET vs PE have, however, shown only excessivetoxicity in the experimental arms without improvement in efficacy. Finaldata from similar trials with topotecan/paclitaxel are awaited.Experimental approaches
A variety of experimental approaches have been tested in SCLC. They includehigh doses of chemotherapy and autologous bone marrow transplantation(BMT), alternating regimens of chemotherapy, and weekly administrationof chemotherapy.High-dose chemotherapy plus BMT Most phase II trials using high dosesof chemotherapy plus BMT appear to show no advantage of the high-doseapproach over standard doses of chemotherapy.Alternating chemotherapy regimens have been used in an attempt to overcomedrug resistance. In randomized trials, alternating chemotherapy regimenshave shown a slight improvement in terms of median survival (4-6weeks) when compared with a single chemotherapeutic regimen but no improvementin long-term survival.PALLIATION OF LOCAL AND DISTANT SYMPTOMSRadiation therapyMany patients with lung cancer have distressing local symptoms at somepoint in their disease course. These symptoms may arise from airway obstructionby the primary tumor, compression of mediastinal structures bynodal metastases, or metastatic involvement of distant organs. Radiationtherapy is effective in palliating most local symptoms, as well as symptoms atcommon metastatic sites, such as bone and brain.Doses In the United States, most radiation oncologists use doses in therange of 30 Gy in 10 fractions for palliative treatment. Data from the UnitedKingdom suggest that similar efficacy without greater toxicity may be achievedwith more abbreviated schedules, such as 17 Gy in 2 fractions 1 week apart orsingle fractions of 11 Gy (see chapter 6, Table 5). Such schedules may facilitatethe coordination of irradiation and chemotherapy and also reduce patienttravel and hospitalization.Endobronchial irradiation with cobalt-60 or iridium-192 has been used topalliate symptoms arising from partial airway obstruction, including cough,dyspnea, and hemoptysis. The dosimetric advantage of being able to delivera high radiation dose to the obstructing endobronchial tumor while sparingadjacent normal structures, such as the lungs, spinal cord, and esophagus,has clear appeal, particularly in the patient whose disease has recurred followingprior external-beam irradiation. Although good rates of palliationhave been reported with endobronchial irradiation, significant complications,including fatal hemoptysis, are seen in 5%-10% of patients. Whether thisrepresents a true treatment complication vs the underlying disease remainsunclear.Other local approaches
Endobronchial irradiation should be considered as only one of several approaches(including laser excision, cryotherapy, and stent placement) thatcan be used in the management of patients with symptomatic airway obstruction,and management should be individualized. All of these approaches aremore suitable for partial rather than complete airway obstruction.Chemotherapy
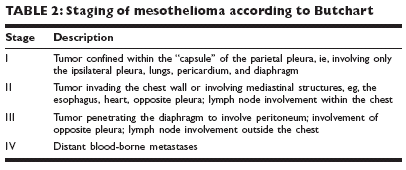
Several recent trials have explored the use of chemotherapy to palliate specificsymptoms in patients with lung cancer. In general, these trials havefound that rates of symptomatic improvement were considerably higher thanobjective response rates and were not dissimilar to symptomatic responserates with local radiation therapy. Chemotherapy in the newly diagnosedpatient is highly palliative for relief of symptoms related to superior venacava syndrome, obstructive lung disease, and painful bony metastases. In thepatient with recurrent disease, irradiation is more commonly associated withsymptomatic relief from these localized problems. Radiation therapy remainsthe standard of care for even chemotherapy-naive patients with spinal cordcompression or symptomatic brain metastasis.Follow-up of long-term survivorsAt present, no standard follow-up protocol exists for patients with curedSCLC or NSCLC. However, at a minimum, long-term follow-up shouldinclude serial physical examinations once the patient has reached the 5-yearmark. Controversy currently exists about the value of utilizing CT scanningor even chest x-rays for the long-term follow-up of these patients.In this vein, retrospective reviews of the literature have revealed that patientswith SCLC appear to have the highest rate of second primary tumordevelopment, as high as 30% over the course of their lifetime, with somestudies reporting annual second primary tumor rates of 5%-10%. Therefore,the concept of chemoprevention appears to have particular merit in thesepatients.MESOTHELIOMAMesotheliomas are uncommon neoplasms derived from the cells lining thepleura and peritoneum. Currently, 2,000-3,000 new cases are diagnosed inthe United States each year.EpidemiologyGender Men are affected five times more commonly than women.Age The median age at diagnosis is 60 years.Etiology and risk factorsAsbestos exposure The relationship between asbestos exposure and diffusepleural mesothelioma was first reported by Wagner, who documented33 pathologically confirmed cases from an asbestos mining region in SouthAfrica. Selikoff and colleagues documented a 300-fold increase in mortalityfrom mesothelioma among asbestos insulation workers in the New Yorkmetropolitan region when compared with the general population. The intervalbetween asbestos exposure and tumor formation is commonly 3-4decades.Asbestos fibers are generally divided into two broad groups: serpentine andamphibole. The latter includes crocidolite, the most carcinogenic form ofasbestos. The inability of phagocytic cells to digest the fiber appears to initiatea cascade of cellular events that results in free-radical generation andcarcinogenesis.DiagnosisPatients with mesothelioma usually seek medical attention while the diseaseis limited to a single hemithorax and commonly complain of dyspnea andpain. Dyspnea results from diffuse growth of the tumor on both the parietaland visceral pleura, which encases the lung in a thick rind. Pain is caused bydirect tumor infiltration of intercostal nerves.Chest x-ray and CT Chest x-ray demonstrates pleural thickening, pleuralbasedmasses, or a pleural effusion. Chest CT scan more accurately portraysthe extent of disease and frequently reveals chest wall invasion, as well aspericardial and diaphragmatic extension.Thoracentesis and thoracoscopy Thoracentesis and pleural biopsy usuallyare sufficient to establish the diagnosis of malignancy, but a thoracoscopic oropen biopsy is often required to provide enough tissue to make an accuratehistologic diagnosis of mesothelioma.Distinguishing mesothelioma from other neoplasms Light microscopy isoften insufficient for differentiating amongmesothelioma, metastatic adenocarcinoma,and sarcoma. Immunohistochemistry andelectron microscopy are frequently necessaryto establish the diagnosis.Although adenocarcinomas stain positive forcarcinoembryonic antigen (CEA), Leu-M1,and secretory component, mesotheliomasstain negative for these markers. Mesotheliomasstain positive for cytokeratin, whereassarcomas do not. Mesotheliomas have characteristicallylong microvilli that are well demonstratedby the electron microscope; adenocarcinomashave short microvilli.PathologyMesotheliomas may contain both epithelial and sarcomatoid elements andare classified by the relative abundance of each component. Epithelial mesotheliomasare most common (50%), followed by mixed (34%) and sarcomatoid(16%) tumors. Survival for the epithelial type is 22 months, comparedwith patients with only 6 months for patients with other types.Staging and prognosisThe most commonly utilized staging system for mesothelioma, that of Butchart,is based on inexact descriptions of the extent of local tumor growth or distantmetastases (Table 2). Other, more detailed staging systems based onTNM criteria have been proposed.Median survival following diagnosis ranges from 9-21 months. Although autopsyseries have demonstrated distant metastases in as many as 50% ofpatients with mesothelioma, death usually results from local tumor growth.TreatmentTreatment rarely results in cure and shouldbe considered palliative.Surgical options include chest tube insertionand pleurodesis to control the pleuraleffusion. Currently, there is renewed interestin aggressive treatment that includesextrapleural pneumonectomy with concomitantresection of the diaphragm and pericardium,followed by chemotherapy and radiotherapy.Subtotal pleurectomy is a less extensivesurgical procedure that debulks themajority of tumor, permits reexpansion ofthe lung, and prevents recurrence of thepleural effusion.Chemotherapy The benefit of chemotherapyfor patients who have unresectablemesothelioma was clarified in a recent randomizedtrial. This study was a single-blind,multicenter, two-arm trial with cisplatin alonein the control arm and cisplatin combinedwith the multitargeted antifolate pemetrexed(Alimta) in the experimental arm. The studywas based on the observation that pemetrexedproduced a 16% objective responserate in previous phase II evaluation. In therandomized trial, patients treated withpemetrexed and cisplatin had an estimated median survival of 12.1 months,as compared with 9.3 months in those treated with cisplatin alone. On thebasis of this survival improvement, the combination of pemetrexed andcisplatin has received an FDA indication for the treatment of unresectablemesothelioma. The same combination is undergoing further evaluation in aneoadjuvant approach in patients with resectable disease.
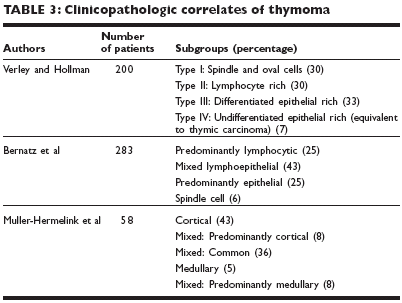
THYMOMAThymoma is a rare mediastinal tumor that occurs mainly in the anterosuperiormediastinum.EpidemiologyGender The tumor affects both sexes equally.Age Thymoma is most often seen in people in the fourth and fifth decades oflife.Etiology and associated syndromesThe etiology of thymoma is unknown, and the risk factors have not beenidentified. Thymoma is a tumor originating within the epithelial cells of thethymus. One-third to one-half of patients present with an asymptomaticanterior mediastinal mass, one-third present with local symptoms (eg, cough,chest pain, superior vena cava syndrome, and/or dysphagia), and one-thirdof cases are detected during the evaluation of myasthenia gravis. Distantmetastases are distinctly uncommon at initial presentation of this tumor.In addition to myasthenia gravis, which occurs in approximately 30% ofpatients with thymoma, a host of paraneoplastic syndromes have been seenin association with thymoma. These other syndromes, which occur in lessthan 5% of patients, include pure red cell aplasia, hypogammaglobulinemia,and a variety of other autoimmune disorders.DiagnosisThe most commonly described symptoms are pleuritic chest pain or discomfort,dry cough, and dyspnea. Physical examination may reveal adenopathy,wheezing, fever, superior vena cava syndrome, vocal cord paralysis, andother paraneoplastic syndromes.Chest x-ray and CT scan A chest x-ray provides an initial basis for diagnosis.The location, size, density, and presence of calcification within the mass canall be determined. Comparison of the film to previously obtained films isusually helpful.Following identification of a mediastinal mass on conventional radiography,contrast-enhanced CT scanning should be performed. CT scanning can differentiatethe cystic form from a solid lesion as well as the presence of fat,calcium, or fluid within the lesion. MRI is increasingly available for use in theevaluation of mediastinal pathology, but it is less frequently utilized than CT.MRI is superior to CT scanning in defining the relationship between mediastinalmasses and vascular structures and is useful in the assessment of vascularinvasion by the tumor.Invasive diagnostic tests CT-guided percutaneous needle biopsy specimensare obtained using fine-needle aspiration techniques and cytologic evaluationor with larger-core needle biopsy and histologic evaluation. Fine-needlespecimens are usually adequate to distinguish carcinomatosis lesions, butcore biopsies may be necessary to distinguish most mediastinal neoplasms.Immunohistochemical techniques and electron microscopy have greatly improvedthe ability to differentiate the cell of origin in mediastinal neoplasms.Most series reported diagnostic yields for percutaneous needle biopsy of70%-100%.Mediastinoscopy is a relatively simple surgical procedure accomplished withthe patient under general anesthesia. It is an adequate approach to the superior,middle, and upper posterior mediastinum, and most series report adiagnostic accuracy of 80%-90%. Anterior mediastinotomy (Chamberlainapproach) provides for direct biopsy of tissue and has a diagnostic yield of95%-100%. Thoracotomy is occasionally necessary to diagnose mediastinalneoplasms, but its indications have been largely supplanted by video-assistedthoracoscopic techniques, which yield 100% accuracy.The most common tumors in the differential diagnosis of an anterior mediastinaltumor are lymphomas and germ-cell tumors. Immunohistochemicalmarkers are helpful to differentiate thymoma from tumors originating fromother cell types.PathologyThree of the most common classification schemes for thymoma are listed inTable 3. Verley and Hollman propose a classification system based on tumorarchitecture, cellular differentiation, and predominant cell type. Bernatz et aldescribe a simpler classification by presenting thymoma based on the percentageof epithelial cells and lymphocytes. In both of these systems, thymomawith a predominance of epithelial cells was associated with a greaterincreased incidence of invasion and a subsequently worse prognosis.
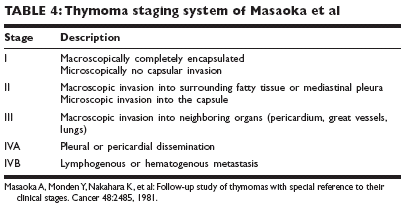
Staging and prognosisThe staging system proposed by Masaoka et al has been widely adopted(Table 4). Stage is an independent predictor of recurrence and long-termsurvival. The 5-year survival rates are 96% for stage I thymoma, 86% for stageII, 69% for stage III, and 50% for stage IV.TreatmentSURGICAL TREATMENT
All patients whose tumors are potentially resectable should undergo surgery.If the patients have evidence of myasthenia gravis, a preoperative consultationwith a clinical neurologist should be considered. The incision of choice isalmost always a median sternotomy, which is quick and easy to make, andprovides excellent exposure to the anterior mediastinum and neck. Althoughthe surgeon is considered the best judge of a tumor's invasiveness, it is oftendifficult to grossly separate invasion from adherence to surrounding tissue.Experience with minimally invasive approaches (such as transcervical thymectomy)is growing; however, until longer term data become available, sternotomyshould still be considered the standard surgical approach.
Complete resection of thymoma has been found to be the most significantpredictor of long-term survival. Several studies have examined the extent ofsurgical resection on survival and disease-free survival rates. In 241 operativecases, Maggi and colleagues found an 82% overall survival rate in those whosetumors underwent complete resection and a 26% survival rate at 7 years inthose undergoing biopsy alone. Other investigators reported similar resultsin surgical patients. Therefore, regardless of stage, tumor resectability is oneof the important predictors of treatment outcome.RADIATION TREATMENT
Thymomas are generally radiosensitive tumors, and the use of radiationtherapy in their treatment is well established. It has been used to treat allstages of thymoma, either before or after surgical resection. General agreementexists regarding the postoperative treatment of invasive thymoma (stagesII and III), whereas the role of irradiation in the treatment of encapsulated(stage I) thymomas is less clear. The value of adjuvant radiation therapy forinvasive thymomas is well documented and should be included in the treatmentregimen regardless of the completeness of tumor resection.CHEMOTHERAPY
Chemotherapy has been used in the treatment of invasive thymomas withincreasing frequency during the past decade (Table 5). The most active agentsappear to be cisplatin, doxorubicin, ifosfamide (Ifex), and corticosteroids.Combination chemotherapy has generally shown higher response rates andhas been used in both neoadjuvant and adjuvant settings and in the treatmentof metastatic or recurrent thymomas. CAP or CAPPr (cyclophosphamide,Adriamycin [doxorubicin], Platinol [cisplatin], and prednisone) regimenshave been used in neoadjuvant and/or adjuvant settings. These regimenshave also been used in recurrent thymoma.
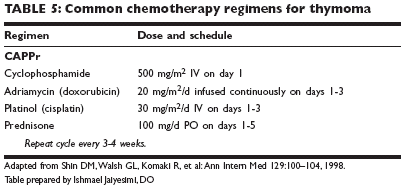
Unresectable thymomaAdvanced-stage (III/IVA) thymomas are usually difficult to remove completely.Multidisciplinary approaches, including induction chemotherapy followedby surgical resection, postoperative radiation therapy, and consolidationchemotherapy, have been reported.Induction chemotherapy consists of cyclophosphamide (500 mg/m2 IV onday 1), doxorubicin (20 mg/m2/d, continuous infusion, on days 1-3), cisplatin(30 mg/m2/d IV on days 1-3), and prednisone (100 mg/d PO on days 1-5),repeated every 3-4 weeks for three courses. Twenty-two evaluable patientswere consecutively treated from 1990 to 2000 in a prospective phase II studyat M. D. Anderson Cancer Center. After induction chemotherapy, 17 of 22patients (77%) had major responses, including three complete responses.Twenty-one patients underwent surgical resection. All patients received postoperativeradiation therapy and consolidation chemotherapy. With medianfollow-up of 50.3 months, overall survival rates at 5 years and 7 years were95% and 79%, respectively. Disease progression-free survival rates were 77%at 5 years and 7 years. The multidisciplinary approaches to unresectablethymoma appear to be promising.
References:
ON SMALL-CELL LUNG CANCER
Auperin A, Arriagada R, Pignon J-P, et al:
Prophylactic cranial irradiation for patientswith small-cell lung cancer in complete remission: Prophylactic Cranial IrradiationOverview Collaborative Group. N Engl J Med 341:476â484, 1999.
Choi N, Herndon II JE, Rosenman J:
Long-term survival data from CALGB 8837:Radiation dose escalation and concurrent chemotherapy (CT) in limited stage small-celllung cancer (LD-SCLC): Possible radiation dose-survival relationship (abstract). ProcAm Soc Clin Oncol 21:298, 2002.
Fried DB, Morris DE, Hensing TA, et al:
Timing of thoracic radiation therapy incombined modality therapy for limited-stage small-cell lung cancer: A meta-analysis(abstract). Lung Cancer 41:S23, 2003.
Janne P, Freidlin B, Saxman S, et al:
The survival of patients treated for limited stagesmall-cell lung cancer in North America has increased during the past 20 years. Proc AmSoc Clin Oncol 20:317, 2001.
Movsas B, Moughan J, Komaki R, et al:
Radiotherapy (RT) Patterns of Care Study(PCS) in lung carcinoma. J Clin Oncol 24:4553-4559, 2003.
Neill HB, Herndon JE, Miller AA, et al:
Randomized phase III intergroup trial(CALGB 9732) of etoposide and cisplatin with or without paclitaxel and G-CSF inpatients with extensive stage small-cell lung cancer (ED-SCLC) (abstract). Proc Am SocClin Oncol 21:293, 2002.
Noda K, Nishawaka Y, Kawahara M, et al:
Irinotecan plus cisplatin compared withetoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85â91,2002.
Ode M, Tsuchiya R, Suzuki K, et al:
Patterns of failure in phase II trial of postoperativeadjuvant cisplatin/etoposide (PE) in patients with completely resected stage I-IIIA smallcelllung cancer (SCLC): The Japanese Clinical Oncology Lung Cancer Study GroupTrial ( JCOG 9101) (abstract). Proc Am Soc Clin Oncol 20:316, 2001.
Schild SE, Brindle JS, Geyer SM, et al:
Long-term results of a phase III trial comparingonce daily radiotherapy (qd RT) with twice daily radiotherapy (bid RT) in limitedstage small-cell lung cancer (LS-SCLC). Int J Radiat Oncol Biol Phys 59:925-927, 2004.
Takada M, Fukuoka M, Kawahara M, et al:
Phase III study of concurrent versussequential thoracic radiotherapy in combination with cisplatin and etoposide for limitedstage small-cell lung cancer: Results of the Japanese Clinical Oncology Group Study9104. J Clin Oncol 20:3054â3060, 2002.
Turrisi A, Kim K, Blum R, et al:
Twice-daily compared with once-daily thoracicradiotherapy in limited small-cell lung cancer treated concurrently with cisplatin andetoposide. N Engl J Med 340:265â271, 1999.
Videtic G, Stitt L:
Patients who smoke during concurrent chemoradiation (ChT/RT)for limited small-cell lung cancer (SCLC) have decreased survival (abstract). Proc AmSoc Clin Oncol 21:295, 2002.
Von Pawel J, Schiller JH, Shepherd FA, et al:
Topotecan versus cyclophosphamide,doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J ClinOncol 17:658â667, 1999.
ON MESOTHELIOMA
Hazarika M, White RM Jr, Booth BP, et al:
Pemetrexed in malignant pleural mesothelioma.Clin Cancer Res 11:982â992, 2005.
ON THYMOMA
Kim ES, Putnam JB, Komaki R, et al:
A phase II study of a multidisciplinary approachwith induction chemotherapy (IC), followed by surgical resection (SR), radiation therapy(RT), and consolidation chemotherapy (CC) for unresectable malignant thymomas:Final report (abstract). Proc Am Soc Clin Oncol 20:310, 2001.