Stage III Lung Cancer: Two or Three Modalities?
Lung cancer is the leading cause of cancer mortality in the United States. A significant number of patients present with disease involving mediastinal lymph nodes. As survival after surgery alone for stage III disease is poor, radiation therapy and chemotherapy have been evaluated in the neoadjuvant and adjuvant settings to improve outcomes. The benefit of adjuvant chemotherapy in the subgroup of patients with N2 disease is uncertain. Small randomized trials enrolling patients with stage III disease have shown a benefit of neoadjuvant chemotherapy over surgery alone. Whether neoadjuvant chemotherapy is superior to adjuvant chemotherapy is under investigation. Furthermore, whether neoadjuvant chemoradiotherapy is superior to neoadjuvant chemotherapy is controversial, and few randomized studies comparing these approaches have been reported. Nevertheless, neoadjuvant chemoradiotherapy appears to be associated with higher rates of resection, higher rates of clearance of mediastinal nodal disease, and better local/regional control. The use of postoperative radiation therapy (PORT) has declined since the publication of the 1998 meta-analysis suggested a detriment in survival with this strategy. However, radiation techniques are improving and emerging data support the use of carefully delivered PORT. Finally, it remains unclear whether surgical resection offers an advantage over definitive chemoradiotherapy alone for stage III disease. In summary, locally advanced NSCLC remains a formidable challenge with few cures, and optimal treatment requires the careful use of surgery, chemotherapy, and radiation therapy.
Lung cancer is the leading cause of cancer mortality in the United States. A significant number of patients present with disease involving mediastinal lymph nodes. As survival after surgery alone for stage III disease is poor, radiation therapy and chemotherapy have been evaluated in the neoadjuvant and adjuvant settings to improve outcomes. The benefit of adjuvant chemotherapy in the subgroup of patients with N2 disease is uncertain. Small randomized trials enrolling patients with stage III disease have shown a benefit of neoadjuvant chemotherapy over surgery alone. Whether neoadjuvant chemotherapy is superior to adjuvant chemotherapy is under investigation. Furthermore, whether neoadjuvant chemoradiotherapy is superior to neoadjuvant chemotherapy is controversial, and few randomized studies comparing these approaches have been reported. Nevertheless, neoadjuvant chemoradiotherapy appears to be associated with higher rates of resection, higher rates of clearance of mediastinal nodal disease, and better local/regional control. The use of postoperative radiation therapy (PORT) has declined since the publication of the 1998 meta-analysis suggested a detriment in survival with this strategy. However, radiation techniques are improving and emerging data support the use of carefully delivered PORT. Finally, it remains unclear whether surgical resection offers an advantage over definitive chemoradiotherapy alone for stage III disease. In summary, locally advanced NSCLC remains a formidable challenge with few cures, and optimal treatment requires the careful use of surgery, chemotherapy, and radiation therapy.
Lung cancer is the leading cause of cancer mortality in the United States. Over 172,000 people were diagnosed with lung cancer in 2005, and most will die of their disease.[1] Non-small-cell lung cancer (NSCLC) accounts for over 80% of cases. Unfortunately, only a minority of patients present with early-stage disease, and the long-term survival of patients with more advanced disease is poor.
Stage III NSCLC encompasses a heterogeneous group of patients, which is reflected in the array of accepted treatment alternatives. These include definitive chemoradiotherapy, preoperative chemotherapy (with or without postoperative RT), and preoperative chemoradiotherapy. The literature does not clearly support one approach over the others. The choice of therapy is often dictated by the extent of local/regional disease, patient performance status, medical comorbidities, and patient/physician preference. In general, patients with bulky, unresectable disease or major comorbidities are managed with definitive chemoradiotherapy, whereas treatment involving surgical resection is offered to patients with more favorable disease.
Historical Perspective
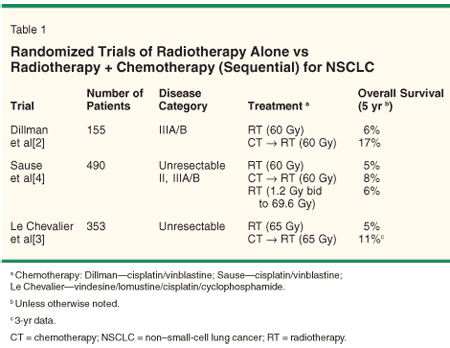
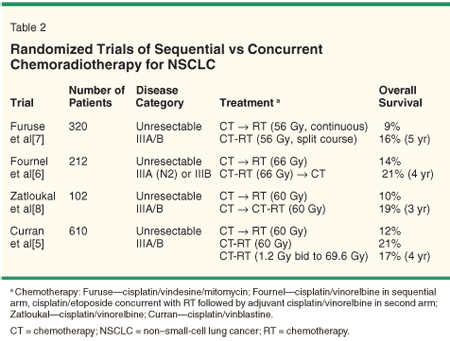
The pattern of care for patients with stage III NSCLC is evolving. Prior to the 1990s, patients with mediastinal disease were generally considered unresectable and were treated with radiation therapy (RT) alone. This strategy yielded a median survival of ~10 months and a disappointing 2-year survival of ~15%, with few long-term survivors. The addition of systemic chemotherapy-initially sequential (Table 1)[2-4] and more recently concurrent (Table 2)[5-8]-was shown in multiple randomized trials to modestly improve survival over RT alone. Even with this advancement, 5-year survival remains less than 20% and local failure is common. This has rekindled interest in surgical resection of select patients with stage III (primarily N2) disease. However, 5-year survival after resection alone for patients with radiographic evidence of N2 disease is < 10%.[9] Therefore, most patients with stage III disease are treated with a multimodality approach of chemoradiotherapy or surgery in combination with neoadjuvant/adjuvant chemotherapy and/or radiotherapy.
Adjuvant Chemotherapy
Several recent studies, largely involving patients with stage IB/II disease, have shown that adjuvant chemotherapy after resection confers a survival benefit over surgery alone (Table 3).[10-13] The only modern positive trial that enrolled stage III patients is the International Adjuvant Lung Cancer Trial (IALT). Among 1,867 patients enrolled in the trial, 479 had N2 disease. Subgroup analysis demonstrated that the hazard ratio for death (death rate with chemotherapy vs without chemotherapy) was lower for patients with stage III disease compared with stage I/II disease, suggesting that the value of chemotherapy is greater for more advanced stages.
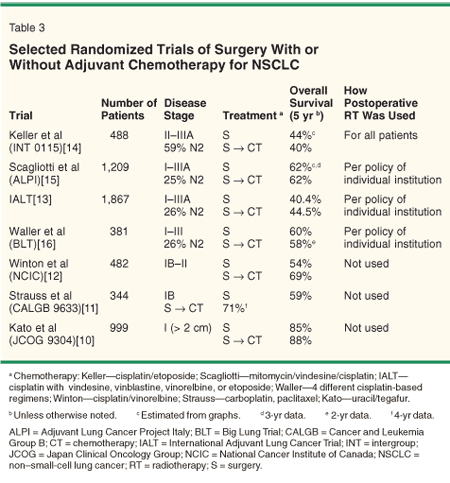
However, other large randomized trials utilizing cisplatin-based regimens, also enrolling stage III patients, have failed to demonstrate a benefit for adjuvant chemotherapy.[14-16] Thus, while it seems clear that adjuvant chemotherapy improves survival in stage IB/II disease, its value in stage III disease is less certain but probably does confer a 5% to 10% survival advantage.
Neoadjuvant Chemotherapy
The administration of chemotherapy prior to surgery (induction therapy) is being used more frequently in patients with stage III lung cancer. This strategy is attractive for several reasons. In patients with advanced local/regional disease, initial chemotherapy may provide tumor regression that facilitates resection. For patients with potentially resectable disease, induction therapy is felt to be better tolerated with higher compliance than adjuvant chemotherapy after thoracotomy. Furthermore, surgical evaluation of the mediastinum after chemotherapy facilitates pathologic assessment of the response to chemotherapy, which may be prognostic and guide future systemic therapies. The local/regional response to induction therapy may also be a surrogate marker for the control of systemic micrometastases. Finally, induction chemotherapy allows for the early treatment of systemic disease.
Randomized Trials
Three small studies reported in the early 1990s,[17-19] each requiring pathologic confirmation of N2 disease, demonstrated that induction chemotherapy followed by resection yielded superior outcomes to surgery alone (Table 4). These studies, as well as the positive findings in the adjuvant setting for patients with stage I/II disease, are often cited to support chemotherapy plus surgery for stage III patients.
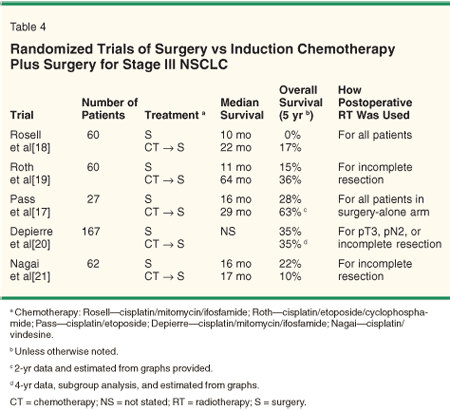
However, more recently reported, larger randomized studies have failed to demonstrate a benefit to induction chemotherapy for stage III NSCLC.[20,21] In the largest such study, conducted by the French Thoracic Cooperative Group,[20] 355 patients (167 with IIIA disease) were randomized to induction and postoperative cisplatin-based chemotherapy vs surgery alone. No difference in overall survival was noted in the subset of patients with stage III disease (relative risk = 1.04, P = .85). Pathologic confirmation of mediastinal involvement was not required in that study. The Japan Clinical Oncology Group conducted a small trial in which 62 patients with pathologic evidence of N2 disease were randomized to surgery alone vs induction cisplatin-based chemotherapy. These investigators found no difference in median survival (16 vs 17 months) or 5-year survival (22% vs 10%, P = .53) between the two arms (Table 4).
Several points about these trials are worth mentioning. First, although the improvements in survival noted in the Roth[19] and Rosell[18] studies are provocative, only 60 patients were enrolled in each study. This increases the probability that prognostic factors were not equally balanced between the two groups. This may explain the unusually poor survival seen after surgical resection alone in the Rosell trial. Second, all of the trials required patients to have tumors deemed resectable prior to the initiation of chemotherapy. Thus, patients with more advanced lesions requiring induction therapy to facilitate resection would not have been eligible. Finally, patterns of failure were not consistently reported, but local/regional recurrence appears to have been a major obstacle.
Postoperative Radiation Therapy
Should thoracic RT remain a component of therapy for patients undergoing induction chemotherapy followed by surgery for stage III lung cancer? The utilization of postoperative RT (PORT) varied among the adjuvant and induction chemotherapy trials mentioned above (Tables 3 and 4). However, the enthusiasm for PORT waned after the 1998 meta-analysis[22] demonstrated a 7% absolute increase in mortality associated with PORT, despite improvements in local/regional control (Table 5).
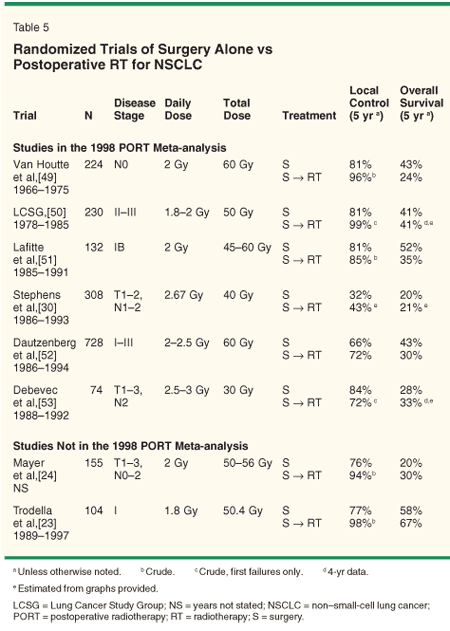
Two points necessitate further discussion. First, the RT techniques utilized in many of the trials would be considered antiquated by contemporary standards. Modern RT treatment planning and delivery is likely associated with less toxicity, thus improving the therapeutic ratio of PORT. Second, none of the trials in the meta-analysis utilized chemotherapy. Improvements in local/regional control afforded by RT is more likely to be meaningful (ie, translate into an improvement in survival) when active systemic therapy is used.
Technique and Toxicity
In the meta-analysis, PORT was associated with a 7% absolute increase in mortality, but the detriment in survival was seen only in patients with stage I/II disease. No advantage or disadvantage to PORT was seen in stage III patients.
These findings can be interpreted as follows: The addition of thoracic RT (using techniques standard at the time) increased mortality by approximately 7%, likely from cardiopulmonary toxicity. This translated into a survival detriment for stage I and II patients, who are at relatively low risk for local/regional recurrence. The patients with stage III disease likely experienced the same rate of RT-induced mortality. Since overall survival in stage III patients was similar with or without RT, the addition of RT likely reduced cancer-specific mortality by 7%. That is, in the patients with stage III disease, the RT-induced deaths offset the reduction in cancer-specific mortality. Therefore, if RT-induced toxicity can be reduced, PORT should improve both cancer-specific and overall survival.
An alternative interpretation of the PORT data is that stage I/II patients experienced more toxicity than stage III patients. This seems unlikely. Nevertheless, it is possible that since the stage III patients have a higher cancer-specific mortality than earlier-staged patients, they have less time at risk to experience such toxicity (ie, it is an issue of competing risks).
• Modern Techniques-As noted, the radiation therapy techniques and doses used in the PORT studies were varied and in many instances antiquated. Modern RT techniques can more accurately direct radiation to the target tissues, thereby reducing the risk of complications, improving the therapeutic ratio. In fact, a recent study from Italy,[23] not included in the 1998 meta-analysis, assessed the utility of PORT directed to small volumes. Patients with pathologic stage I disease were randomized to receive or not receive PORT (50.4 Gy) to the bronchial stump and ipsilateral hilum using three-dimensional treatment planning. This modest-sized study of 104 patients demonstrated a statistically-significant improvement in local control (98% vs 77%, P < .01) and overall survival at 5 years (67% vs 58%, P = .048).
In another randomized study reported in 1997 from Austria (also not included in the PORT meta-analysis), 155 patients were randomized to receive or not receive PORT (50-56 Gy). Modern RT machines and three-dimensional treatment planning appeared to improve 5-year recurrence-free survival (27% vs 16%, P = .07) and perhaps overall survival (30% vs 20% at 5 years, P = NS) without any severe late complications (Table 5).[24] The use of modern treatment planning should reduce the risks associated with RT[25].
• Parallel Clinical Situation-The current controversy regarding PORT for lung cancer is analogous to what was encountered with postmastectomy RT for breast cancer. Several individual trials from the 1970s as well as a meta-analysis demonstrated that postmastectomy RT reduced the risk of dying from breast cancer, but increased the risk of cardiovascular death.[26] The magnitude of these effects was similar, yielding no overall gain from RT. The use of modern RT machines and techniques reduced the rate of treatment-associated mortality. Subsequent studies showed an improvement in local/regional control-and a concomitant improvement in overall survival-with postmastectomy RT.[27-29] The same is likely true for NSCLC. Improved dose delivery to the regions at risk, minimizing dose to the surrounding heart and lungs, should improve the therapeutic ratio.
Importance of Chemotherapy
General oncology principles from other disease sites have bearing on the management of NSCLC. RT clearly improves local control over surgery alone in a host of malignancies including cervical, endometrial, rectal, head and neck, breast, and prostate cancer. A common finding is an improvement in local control with RT alone, but an improvement in local control and survival when RT is combined with chemotherapy. This should not be surprising. Cancer is often a systemic illness. RT is a local therapy. Local control will only translate into a survival benefit if distant micrometastases are treated. In patients with local/regional and systemic disease burdens, the ability of RT to improve survival will increase as the efficacy of systemic treatment increases.
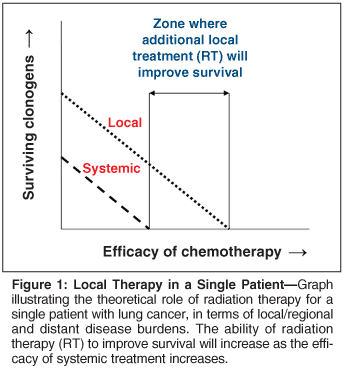
Figure 1 illustrates the theoretical situation for a single patient, and Figure 2 illustrates the situation for a population of patients with a variable degree of local/regional and systemic disease burdens. Theoretically, if systemic therapy becomes extremely effective, it may provide adequate systemic and local/regional control. This might be the case for diseases such as lymphoma or germ cell tumors, but systemic therapy for NSCLC is less effective.
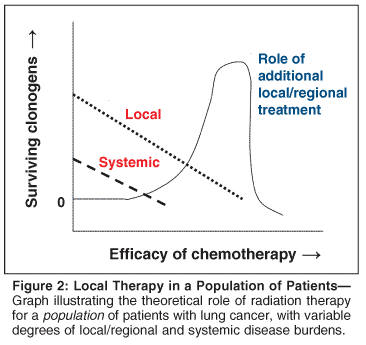
Cancers of the rectum and breast are examples of malignancies in which the ability of local therapy (RT) to affect survival is modified by systemic therapy. Multiple studies have demonstrated an improvement in local control with pelvic RT after radical surgery for rectal cancer.[31-33] This improvement did not readily translate into a survival benefit until more effective chemotherapy became available.[34] The same pattern was seen in breast cancer. Before active systemic chemotherapy, postmastectomy RT consistently improved local control, but no clear benefit in overall survival was seen.[35] With improved radiation techniques and the advent of effective chemotherapy, postmastectomy RT was found to improve overall survival.[27-29]
None of the trials in the PORT meta-analysis utilized systemic chemotherapy. Unfortunately, two trials randomizing patients with stage III NSCLC postresection to chemotherapy vs chemotherapy and RT were closed due to poor accrual (CALGB 9734 and NCCTG 88-24-53). Though the results of the meta-analysis should remind us of the potential toxicities of treatment, PORT should not be abandoned. Keep in mind that RT did improve local/regional control in most of the studies, and local/regional control must be achieved to cure the patient. As systemic therapy for NSCLC improves, the importance of local control will only become greater. Carefully delivered postoperative RT probably does some good.
Neoadjuvant Chemoradiotherapy
Treatment of stage III NSCLC patients with induction chemotherapy alone, followed by resection of grossly-evident disease in the mediastinal lymph nodes, is associated with a 3 50% risk of local/regional recurrence.[17,36] Such high rates of local failure should not be a surprise. Microscopic deposits of tumor likely infiltrate throughout the mediastinal lymphatic network, making a curative en bloc "cancer resection" impractical. At present, mediastinal irradiation is the only realistic means of treating the mediastinal lymphatics.
Randomized Trials
An alternative approach to postoperative RT is to combine RT with induction chemotherapy. Three randomized trials have been presented in abstract form comparing induction chemotherapy to induction chemoradiotherapy (Table 6). A Brazilian study[37] randomized 96 patients with stage IIIA (N2) or T4 disease to induction cisplatin-based chemotherapy vs a different cisplatin-based regimen with RT (30 Gy in 2-Gy fractions). The addition of RT increased the resection rate (31% vs 52%, P = .03) and freedom from disease progression (21% vs 40%, P = .04) with a median follow-up of 3 years. The effect on overall survival was not reported.
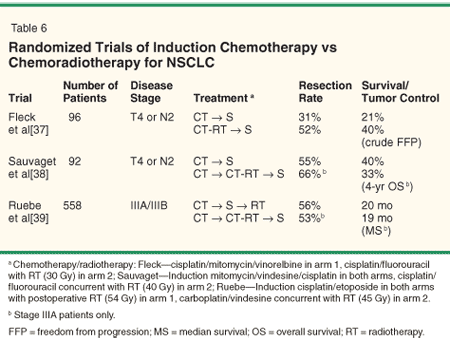
A study from France[38] randomized 92 patients with stage IIIA or T4 disease to two cycles of induction cisplatin-based chemotherapy vs the same chemotherapy followed by concurrent RT (40 Gy in 6 weeks, split course) and chemotherapy regimens with subsequent resection. In the stage IIIA patients, the addition of RT led to higher response rates (55% vs 75%) and resection rates (55% vs 66%), but no differences in median survival (19 vs 18.5 months). Long-term actuarial survival rates were not reported.
Finally, the German Lung Cancer Cooperative Group[39] randomized 558 patients with IIIA or IIIB disease to three cycles of cisplatin/etoposide vs the same regimen followed by RT (1.5 Gy bid to 45 Gy) with concurrent carboplatin/vindesine and subsequent surgery. All patients in the induction chemotherapy-alone arm received postoperative RT. No significant differences were noted in response rates, complete resection rates, or survival.
• Study Limitations-Numerous limitations of these trials hinder a fair assessment of the role of radiation therapy in the induction phase of treatment. First, both the Brazilian and French trials were small and clearly underpowered to detect meaningful differences in outcomes. Second, to distinguish the benefit of radiation therapy (or lack thereof), it would be ideal to utilize an identical chemotherapy regimen in each treatment arm, so that the only difference would be the addition of radiotherapy. Each of the randomized trials utilized different chemotherapy regimens between the two treatment arms, often administering additional cycles of chemotherapy in the patients receiving radiotherapy.
Third, the dose of RT was perhaps too low in the Brazilian and French trials (30 and 40 Gy, respectively). Finally, none of the trials have been reported in peer-reviewed literature. A joint Radiation Therapy Oncology Group (RTOG)-Southwest Oncology Group (SWOG) study is currently comparing induction chemotherapy with cisplatin and docetaxel (Taxotere) with the same chemotherapy administered concurrently with thoracic radiotherapy (50 Gy). All patients will receive adjuvant docetaxel.
• Potential Advantages-Given the limitations of the aforementioned randomized trials, an analysis of phase II studies utilizing induction chemotherapy or induction chemoradiotherapy seems justified (Table 7). Induction chemoradiotherapy has multiple potential advantages over chemotherapy alone. First, rates of mediastinal sterilization appear to be higher when radiation therapy is included in the induction regimen. The reported rates of mediastinal sterilization range from 17% to 53%[17,18,36,40,41] with chemotherapy alone vs 24% to 79%[42-45] with preoperative chemoradiotherapy. Mediastinal sterilization has been shown to be a major prognostic factor for survival in multiple studies.[36,42,44,45] Whether this is a cause-and-effect phenomenon or simply a favorable phenotype is unclear.
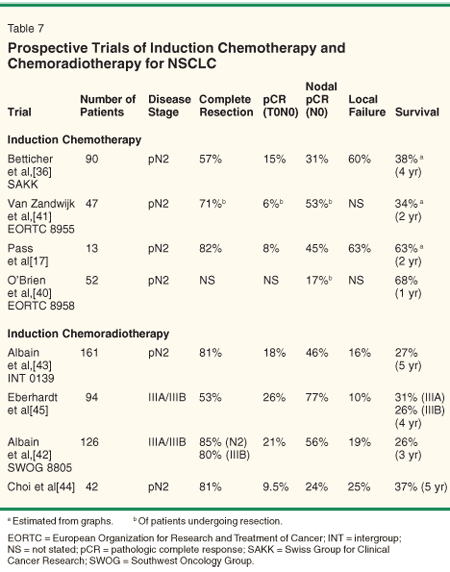
Second, local/regional control may be higher with induction chemoradiotherapy than with chemotherapy alone. Patterns of failure are inconsistently reported, and often only the first site of failure is scored. Thus, actuarial local/regional failures rates are probably higher than reported. The high rate of local/regional failure after induction chemotherapy alone argues that this is not an optimal treatment strategy. Finally, achieving an R0 resection may be superior with a combined-modality approach over chemotherapy alone. Such comparisons need to be made with caution, as these are not randomized comparisons. Nevertheless, they are consistent with the limited randomized data mentioned previously.
Is Surgery Necessary?
It remains unclear whether surgery is an essential component of therapy in stage III (N2) disease. Local failure after concurrent chemoradiotherapy has ranged from 30% to 40% in randomized trials[6-8] and as high as 80% in series systematically assessing results for local control.[46] Approximately 50% of such failures occur without evidence of distant metastases. In theory, surgical resection of residual disease may facilitate local control and improve survival.
Randomized Trials
Four randomized trials have been reported comparing a nonsurgical approach with a surgical regimen for patients with stage III NSCLC (Table 8). Two small trials showed no advantage for surgery[47,48] over radiation therapy with or without chemotherapy.
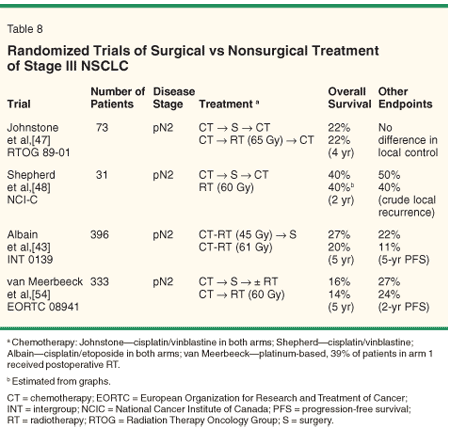
The Intergroup 0139 trial[43] randomized 396 patients with T1-3, pN2 disease to induction chemotherapy (cisplatin and etoposide) and RT (45 Gy) followed by resection vs definitive radiation therapy (61 Gy) with concurrent chemotherapy. All patients were required to have technically resectable disease. The surgical arm had a higher progression-free survival rate (22% vs 11%, P = .017) and a trend toward improved 5-year overall survival (27% vs 20%, P = .10). The differences in progression-free survival were greater than for overall survival due to excess treatment-related mortality in the surgical arm (8% vs 2%). The surgical mortality was especially high (> 20%) in patients undergoing a pneumonectomy.[43] Thus, definitive chemoradiotherapy may be the preferred approach (rather than surgery) in patients requiring pneumonectomy.
Finally, a European Organization for Research and Treatment of Cancer study reported at the 2005 annual meeting of the American Society of Clinical Oncology randomized 333 patients to surgical resection vs RT after induction platinum-based chemotherapy. The investigators found no statistical difference in progression-free or overall survival between the two arms.
Conclusions
Lung cancer is an aggressive disease with a high propensity for local/regional and distant spread. Given the poor prognosis of patients with stage III disease, the judicious use of surgery, radiation therapy, and chemotherapy should be considered for all patients. The optimal timing and integration of each modality remains unclear.
For patients with advanced local disease requiring requiring maximal shrinkage to facilitate resection, it would seem wise to utilize both RT and chemotherapy in the induction phase of treatment. For the remaining patients, we advocate enrollment in the RTOG-SWOG study (RTOG 0412/SWOG 0332), which will help clarify whether induction chemoradiotherapy is superior to chemotherapy alone. Finally, many patients with stage III disease are likely best served with definitive chemoradiotherapy without resection-in particular, those who would require a pneumonectomy and/or those with macroscopic involvement of multiple mediastinal lymph node stations.
Disclosures:
Dr. Kelsey is a speaker for and has received a research grant from Varian Medical Systems.
References:
1. Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. CA Cancer J Clin 55:10-30, 2005.
2. Dillman RO, Herndon J, Seagren SL, et al: Improved survival in stage III non-small-cell lung cancer: Seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 88:1210-1215, 1996.
3. Le Chevalier T, Arriagada R, Quoix E, et al: Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: First analysis of a randomized trial in 353 patients. J Natl Cancer Inst 83:417-423, 1991.
4. Sause W, Kolesar P, Taylor SI, et al: Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 117:358-364, 2000.
5. Curran W, Scott C, Langer CJ, et al: Long-term benefit is observed in a phase III comparison of sequential versus concurrent chemo-radiation for patients with unresected stage III NSCLC (abstract 2499). Proc Am Soc Clin Oncol 22:621, 2003.
6. Fournel P, Robinet G, Thomas P, et al: Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 23:5910-5917, 2005.
7. Furuse K, Fukuoka M, Kawahara M, et al: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17:2692-2699, 1999.
8. Zatloukal P, Petruzelka L, Zemanova M, et al: Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: A randomized study. Lung Cancer 46:87-98, 2004.
9. Andre F, Grunenwald D, Pignon JP, et al: Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 18:2981-2989, 2000.
10. Kato H, Ichinose Y, Ohta M, et al: A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350:1713-1721, 2004.
11. Strauss G, Herndon J, Maddaus M: Randomized clinical trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in stage IB non-small cell lung cancer (NSCLC): Report of Cancer and Leukemia Group B (CALGB) protocol 9633 (abstract 7019). J Clin Oncol 22(suppl):621s, 2004.
12. Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352:2589-2597, 2005.
13. Arriagada R, Bergman B, Dunant A, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351-360, 2004.
14. Keller SM, Adak S, Wagner H, et al: A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med 343:1217-1222, 2000.
15. Scagliotti GV, Fossati R, Torri V, et al: Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J Natl Cancer Inst 95:1453-1461, 2003.
16. Waller D, Peake MD, Stephens RJ, et al: Chemotherapy for patients with non-small cell lung cancer: The surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 26:173-182, 2004.
17. Pass HI, Pogrebniak HW, Steinberg SM, et al: Randomized trial of neoadjuvant therapy for lung cancer: Interim analysis. Ann Thorac Surg 53:992-998, 1992.
18. Rosell R, Gomez-Codina J, Camps C, et al: Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: A 7-year assessment of a randomized controlled trial. Lung Cancer 26:7-14, 1999.
19. Roth JA, Atkinson EN, Fossella F, et al: Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 21:1-6, 1998.
20. Depierre A, Milleron B, Moro-Sibilot D, et al: Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol 20:247-253, 2002.
21. Nagai K, Tsuchiya R, Mori T, et al: A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 125:254-260, 2003.
22. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet 352:257-263, 1998.
23. Trodella L, Granone P, Valente S, et al: Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: Definitive results of a phase III randomized trial. Radiother Oncol 62:11-19, 2002.
24. Mayer R, Smolle-Juettner FM, Szolar D, et al: Postoperative radiotherapy in radically resected non-small cell lung cancer. Chest 112:954-959, 1997.
25. Machtay M, Lee JH, Shrager JB, et al: Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J Clin Oncol 19:3912-3917, 2001.
26. Haybittle JL, Brinkley D, Houghton J, et al: Postoperative radiotherapy and late mortality: Evidence from the Cancer Research Campaign trial for early breast cancer. BMJ 298:1611-1614, 1989.
27. Overgaard M, Hansen PS, Overgaard J, et al: Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 337:949-955, 1997.
28. Overgaard M, Jensen MB, Overgaard J, et al: Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353:1641-1648, 1999.
29. Ragaz J, Jackson SM, Le N, et al: Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337:956-962, 1997.
30. Stephens RJ, Girling DJ, Bleehen NM, et al: The role of post-operative radiotherapy in non-small-cell lung cancer: A multicentre randomised trial in patients with pathologically staged T1-2, N1-2, M0 disease. Medical Research Council Lung Cancer Working Party. Br J Cancer 74:632-639, 1996.
31. Treurniet-Donker AD, van Putten WL, Wereldsma JC, et al: Postoperative radiation therapy for rectal cancer. An interim analysis of a prospective, randomized multicenter trial in The Netherlands. Cancer 67:2042-2048, 1991.
32. Fisher B, Wolmark N, Rockette H, et al: Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: Results from NSABP protocol R-01. J Natl Cancer Inst 80:21-29, 1988.
33. Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med 312:1465-1472, 1985.
34. Krook JE, Moertel CG, Gunderson LL, et al: Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 324:709-715, 1991.
35. Fowble B: Postmastectomy radiation: Then and now. Oncology (Williston Park) 11:213-243 (incl discussion), 1997.
36. Betticher DC, Hsu Schmitz SF, Totsch M, et al: Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: A multicenter phase II trial. J Clin Oncol 21:1752-1759, 2003.
37. Fleck J, Camargo J, Godoy D, et al: Chemoradiation therapy (CRT) versus chemotherapy (CT) alone as a neoadjuvant treatment for stage III non-small cell lung cancer (NSCLC): Preliminary report of a phase III prospective randomized trial (abstract 1108). Proc Am Soc Clin Oncol 12:333, 1993.
38. Sauvaget J, Rebischung J, Vannetzel J: Phase III study of neo-adjuvant MVP versus MVP plus chemoradiotherapy in stage III NSCLC (abstract 1935). Proc Am Soc Clin Oncol 19:495a, 2000.
39. Ruebe C, Riesenbeck D, Semik M, et al: Neoadjuvant chemotherapy followed by preoperative radiochemotherapy (hfRTCT) plus surgery or surgery plus postoperative radiotherapy in stage III non-small cell lung cancer: Results of a randomized phase III trial of the German Lung Cancer Cooperative Group. Int J Radiat Oncol Biol Phys 60:S130, 2004.
40. O'Brien ME, Splinter T, Smit EF, et al: Carboplatin and paclitaxol (Taxol) as an induction regimen for patients with biopsy-proven stage IIIA N2 non-small cell lung cancer. an EORTC phase II study (EORTC 08958). Eur J Cancer 39:1416-1422, 2003.
41. Van Zandwijk N, Smit EF, Kramer GW, et al: Gemcitabine and cisplatin as induction regimen for patients with biopsy-proven stage IIIA N2 non-small-cell lung cancer: A phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group (EORTC 08955). J Clin Oncol 18:2658-2664, 2000.
42. Albain KS, Rusch VW, Crowley JJ, et al: Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 13:1880-1892, 1995.
43. Albain KS, Swann RS, Rusch VR, et al: Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA (pN2) non-small cell lung cancer (NSCLC): Outcomes update of North American Intergroup 0139 (RTOG 9309) (abstract 7014). J Clin Oncol 23(16S):624s, 2005.
44. Choi NC, Carey RW, Daly W, et al: Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J Clin Oncol 15:712-722, 1997.
45. Eberhardt W, Wilke H, Stamatis G, et al: Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: Mature results of a phase II trial. J Clin Oncol 16:622-634, 1998.
46. Arriagada R, Le Chevalier T, Quoix E, et al: ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: Effect of chemotherapy on locally advanced non-small cell lung carcinoma: A randomized study of 353 patients. GETCB (Groupe d'Etude et Traitement des Cancers Bronchiques), FNCLCC (Federation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys 20:1183-1190, 1991.
47. Johnstone DW, Byhardt RW, Ettinger D, et al: Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2): Final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 54:365-369, 2002.
48. Shepherd FA, Johnston MR, Payne D, et al: Randomized study of chemotherapy and surgery versus radiotherapy for stage IIIA non-small-cell lung cancer: A National Cancer Institute of Canada Clinical Trials Group Study. Br J Cancer 78:683-685, 1998.
49. Van Houtte P, Rocmans P, Smets P, et al: Postoperative radiation therapy in lung caner: A controlled trial after resection of curative design. Int J Radiat Oncol Biol Phys 6:983-986, 1980.
50. Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. The Lung Cancer Study Group. N Engl J Med 315:1377-1381, 1986.
51. Lafitte JJ, Ribet ME, Prevost BM, et al: Postresection irradiation for T2 N0 M0 non-small cell carcinoma: A prospective, randomized study. Ann Thorac Surg 62:830-834, 1996.
52. Dautzenberg B, Arriagada R, Chammard AB, et al: A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d'Etude et de Traitement des Cancers Bronchiques. Cancer 86:265-273, 1999.
53. Debevec M, Bitenc M, Vidmar S, et al: Postoperative radiotherapy for radically resected N2 non-small-cell lung cancer (NSCLC): Randomised clinical study 1988-1992. Lung Cancer 14:99-107, 1996.
54. van Meerbeeck J, Kramer G, van Schil P,et al: A randomized trial of radical surgery (S) versus thoracic radiotherapy (TRT) in patients with stage IIIA-N2 non-small cell lung cancer (NSCLC) after response to induction chemotherapy (EORTC 08941) (LBA7015). J Clin Oncol 23(16S):624s, 2005.