PERSPECTIVE
Elizabeth S. Constance, MD discusses fertility preservation for AYAs at www.Cancernetwork.com/AYA_fertility_perspective
ABSTRACT Adolescents and young adults (AYAs) with cancer constitute approximately 70,000 patients diagnosed each year. Survival rates for AYAs with cancer have increased steadily in recent decades due to improvements in therapeutic regimens and early detection. Given the large and growing number of AYA cancer survivors, additional research is needed on the immediate and long-term psychosocial support required for this population including family planning and fertility. Fertility and fertility preservation in female AYAs, in particular, is historically understudied and has psychologically relevant ramifications distinct from male AYAs. Decision science can contribute to this area of oncological care and has implications for clinical encounters and research concerning female AYA patients with cancer. Patient-centered care and shared decision-making that integrates recent research regarding fertility preservation in the context of cancer treatment can improve outcomes for AYA cancer survivors.
Payne is a doctoral student, Department of Psychology, Stonyal student, Department of Psychology, Stony Brook University, Stony Brook, New Yorook University, Stony Br Book, New York.rk.

Flowers is chair of the Department of Lymphoma/Myeloma at the University of Texas MD Anderson Cancer Center.

Allen is assistant professor, Department of Hematology and Medical Oncology, Emory University Winship Cancer Institute, Atlanta, Georgia.

Adolescents and young adults (AYAs) with cancer constitute approximately 70,000 patients diagnosed each year,1 accounting for about 5% of cancer diagnoses.2 AYAs are classified as patients diagnosed between the ages of 15 and 39 and are biologically3 and psychosocially4 distinct from younger and older age groups. Survival rates for AYAs with cancer have increased steadily in recent decades, primarily due to improved therapeutic regimens.5 Currently, AYAs across all cancers have a 5-year survival rate of approximately 80%.6 Given the number of AYA cancer survivors, researchers have begun to study the psychosocial needs of this growing population, including disruption of education and career development, employment, substance abuse, family planning, and fertility.7
Cancer treatments are detrimental to reproductive functioning including fertility. However, fertility in female AYAs is historically understudied and has psychologically relevant ramifications distinct from male AYAs.8 Until recently, there were few fertility preservation options available for women. Although they are becoming more common, fertility preservation procedures for women are more invasive, more expensive, and delay cancer treatment much longer than fertility preservation procedures for men.9,10 In addition, many providers do not prioritize and are hesitant to discuss fertility preservation with female AYA patients.11 Uncertain risks and lack of information about future fertility, coupled with little time to make a potentially life-changing decision about pursuing fertility preservation before beginning cancer treatment, creates a complex clinical and psychosocial decision-making scenario for AYA women diagnosed with cancer.12
Decision science, in the context of medical treatment decision-making, is understudied in oncological care. As medical researchers continue to improve survival rates and fertility options for this population of patients with cancer, the psychological study of treatment decision-making in the context of fertility is needed. This review aims to summarize the work in this area to date and to define future areas for scientific inquiry.
Oncofertility is the study of fertility preservation in the context of cancer diagnosis, treatment, and survival.11,13 In women, an individual’s ovarian reserve, or number of oocytes, decreases over time. For fertility and normal endocrine function to be maintained, the menstrual cycle routinely develops oocytes from the ovarian reserve, secreting essential hormones in the process. Disturbance of the ovarian reserve, especially in younger women, has immediate and long-term effects on health and fertility.13
For AYA women, the cancers with the highest incidence rates are breast cancer, thyroid cancer, and lymphoma.14(Table 1) A growing body of research studies suggests that AYAs have biological and genetic differences from pediatric and older adult patients with cancer.2 For example, AYA breast patients with cancerare more likely to present with genetic mutations, making the cancer more aggressive and harder to treat than in older adult patients. Triple-negative breast cancer is overrepresented in the AYA patient population. Thyroid cancer and lymphomas in AYAs are also more likely to exhibit mutational characteristics than in older adult patients. Due to this, and to their relative health compared with older adults, AYA patients with cancer are more likely to receive aggressive therapy regimens.14
Table 1. Age-Adjusted Rate of Cancer Incidence for Common AYA Cancers in Women by Age Group
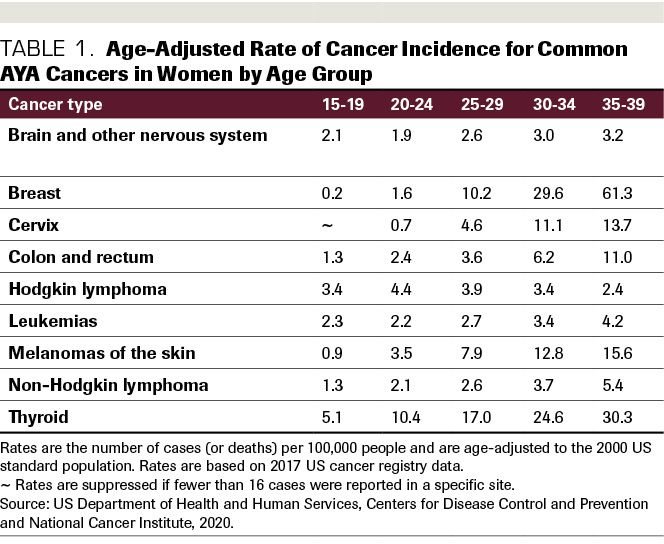
The common treatments for AYA cancer, chemotherapy and radiation, often result in significantly impaired fertility in women and men.15(Table 2) (A notable exception is the frontline therapy for Hodgkin lymphoma, the ABVD [doxorubicin, bleomycin, vinblastine, dacarbazine]) regimen.16 In addition, there are non–reproductive health issues that may result in the long term due to dysregulation of the endocrine system, including the cardiovascular system and bone health.13 The mechanisms for this are still being studied, as better treatments are relatively new. Although dependent on the type and duration of treatment cycles, chemotherapy regimens designed to destroy cancer cells also are damaging to ovarian reserves. Regarding radiation, exposure to even small amounts lead to premature ovarian failure.13
Advances in fertility preservation methods have created options for young women that were previously unavailable. With the emerging interest in these methods and concerns about the associated psychological ramifications, expert guidance on the effectiveness and best practices for implementation in clinics were needed. The International Network on Cancer, Infertility, and Pregnancy (INCIP) and the European Society of Gynecological Oncology (ESGO) are currently tasked with creating recommendations for the presentation of fertility preservation options and standards of care for AYA cancer patients.15 Currently, the options available to women who are able and willing to preserve fertility before treatment are oocyte or embryo cryopreservation (slow freezing of tissue), ovarian transposition (relocation of ovaries within the body), or administration of follicle-protecting agents.17 Several experimental options are also emerging, including the use of artificial ovaries and in vitro follicle cultures, which are currently being studied in nonhuman primates.15 Each method comes with advantages and disadvantages, which are outside the scope of this review.
Table 2. Impact of Common Cancer Treatments on Fertility in AYA Women
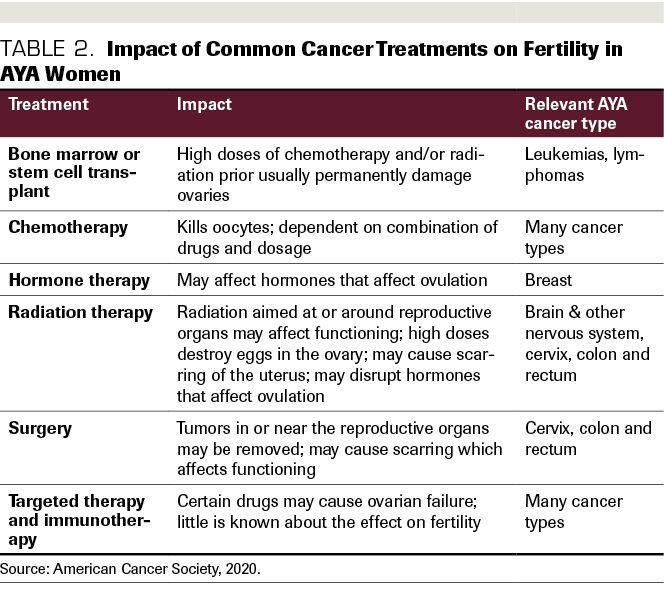
The potential side effects and risk of failure of fertility preservation efforts, coupled with potential uncertainty about future family plans, often makes the decision about whether to pursue
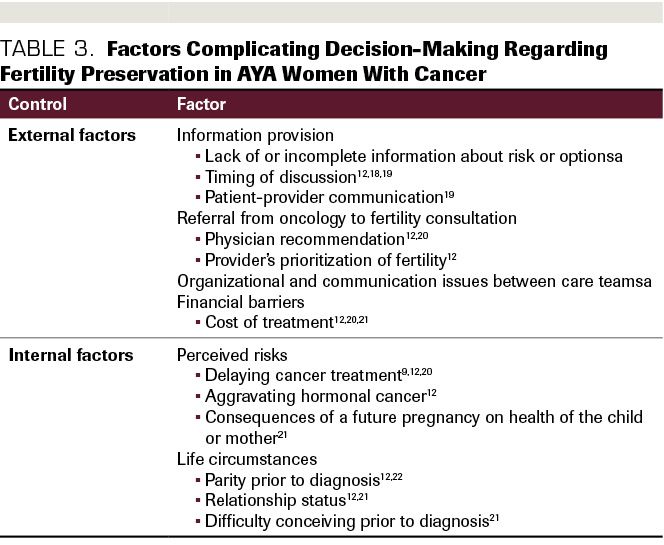
pretreatment preservation difficult.12(Table 39,12, 18-22) Pursuing fertility preservation also delays cancer treatment,9 contributing to the pressure and uncertainty already present in the decision-making process. In addition, the expense and time needed to pursue these methods serve as barriers to choosing this option at all. The stress of dealing with an unexpected cancer diagnosis, and urgency to begin treatment for more aggressive cases, may also lessen the attention young women and their families give to fertility preservation before beginning treatment.12
Historically, AYAs were not treated as a distinct patient population. Oncologists treating children and teenagers were trained in pediatric oncology, but at the age of 18 patients became adults and were treated similarly to all older adults. As the psychosocial needs of young adults gained more attention, the AYA subgroup became clinically distinct.4 Still, fragmentation of care and lack of standard protocols prevent many AYA patients with cancer and survivors from receiving the specialized care most relevant to them, including fertility planning.4,7 Developmentally, AYAs have distinct needs from children and older adults. The adult brain is not fully formed until around the age of 30, and the effects of cancer treatment on AYA development are understudied.14 AYAs also are in a phase of their life where they may not have developed the coping skills to deal with traumatic disruptions to life goals such as a cancer diagnosis. In addition, considering the stress of a potential lack of education attainment, financial instability, and the side effects of treatment, AYA cancer survivors report difficulty in creating and maintaining meaningful sources of social support.23
Table 3. Factors Complicating Decision-Making Regarding Fertility Preservation in AYA Women With Cancer
Given the stressful context of cancer diagnosis, treatment, and survivorship specific to AYA patients with cancer, making a decision about family planning before treatment is a difficult stressor to cope with among many other difficult stressors. Additionally, it is important to recognize individual differences in coping with the potential loss of fertility due to cancer treatment.
For AYA patients with cancer, fertility is the second most important factor when choosing a treatment, surpassed only by survival.13 Decision science incorporates several fields and methodologies that examine how individuals make decisions. In health care, medical decision-making research frequently utilizes psychological inquiry into individual values, interpersonal dynamics, and structural barriers affecting decisions about prevention and treatment of health conditions.24
Patient-Centered Care and Shared Decision-Making
Over the past few decades, patient-centered care has grown from a new buzzword in health care to a paradigm with a growing bed of evidence and funding. Patient-centered care refers to a way of delivering medical care that encourages active collaboration between patients, their families and caregivers, and providers.25 The incorporation of patient-centered care shifts the aim of medical practitioners from acting upon a patient to acting with a patient. In this model of care, the patient is meant to be viewed as a consumer with values and preferences.26
Shared decision-making is a core value of patient-centered care. It is an iterative process of communication between a provider or care team and a patient about treatment options and risks, as well as the patient’s personal preferences and values. When implemented, shared decision-making between patients and providers reduces decisional conflict, feelings of being uninformed, depersonalization of the individual, passivity, and indecision - all of which are detrimental to well-being and satisfaction.27,28
Patients may also bring additional challenges to their treatment decision-making, including cognitive factors, such as limited knowledge of treatment options, insufficient numeracy, and health literacy to understand risk and probability of various outcomes;29,30 and emotional factors, such as decisional conflict and uncertainty.24 As shared decision-making becomes more prevalent in cancer treatment decision-making, addressing the barriers to informed decision-making in clinical populations emerges as an essential factor for improving patient-centered outcomes.31,32
Concepts such as decisional conflict, decisional regret, and values clarification are engrained in the shared decision-making literature28 and apply to the dilemma female AYA patients with cancer face regarding fertility preservation. In one qualitative study, values clarification exercises administered to adolescent girls facing fertility loss indicated that all of the participants strongly desired the ability to conceive children in the future, although participants had different coping styles for dealing with the loss of this ability.8 The psychological understanding of the mechanisms of decision-making is also relevant to cancer decision-making specifically, illuminating the need for understanding the cognitive and emotional factors relevant to the components of informed, shared decision-making.24
A recent review12 of factors that make fertility preservation decisions difficult for women found several internal and external factors at play. Externally, lack of information, improper timing of information, and poor patient-provider communication prevent women from making informed decisions. In addition, uncertainty about negative outcomes of fertility preservation procedures makes decision-making difficult, including the effect of needing to delay cancer treatment to perform the procedure and potential complications that could arise with post treatment pregnancy. For women with cancers of the reproductive system, the risks of aggravating a malignancy with further reproductive system procedures are also a factor to take into consideration. Fragmentation of care at the organizational level prevents many women from having the full attention of a provider advocate to help make sense of the factors important to this decision, and as a result, some women may choose not to pursue fertility preservation and complicate an already complex provider care team. The researchers also found that for providers not specialized in AYA psychosocial issues, fertility preservation is not a priority and may be conveyed as such in the presentation of treatment options for AYA women. Based on these findings, there exists a clear need for organizational- and interpersonal-level intervention at the point of fertility preservation decision-making. Thorough, clear presentation of the options available to women and associated risks is essential.
Elizabeth S. Constance, MD discusses fertility preservation for AYAs at www.Cancernetwork.com/AYA_fertility_perspective
Internally, there are key factors women must address when making this decision.12 When faced with an unexpected cancer diagnosis, AYAs are faced with significant decisions with implications for their futures, and these decisions must usually be made quickly. For many, family planning is not something they may have expected to think about for several years. In this situation, it is not uncommon for women to enter what the review describes as “survival mode,” or the immediate prioritization of survival. As a result, the impact on fertility may not be at the forefront of a treatment decision. Reinforced by oncologists concerned about disease progression, AYAs tend to opt for the more aggressive regimens that ensure the highest chance of survival. In addition to this dilemma, women must weigh the costs and benefits of financially committing to such a procedure, as well as reckon with the potential impact on their personal lives and relationship dynamics.33
Multidisciplinary programs that target the physical and psychosocial needs of AYA patients with cancer hold promise for significantly improving the quality of life of patients and may help patients emotionally cope with cancer. One such program increased fertility preservation referrals and procedures by designating a multidisciplinary program to provide education about fertility preservation to eligible patients.34 A recent systematic review also found that women who receive targeted fertility preservation counseling exhibit improved coping over time, and reduced regret and dissatisfaction with care long-term.35 Guidelines for how to discuss fertility preservation with AYA patients with cancer are a recent development, and little research has yet to be done on the long-term effects of implementing satisfactory interventions to improve decision-making. Within the context of shared decision-making, fertility preservation conversations should be incorporated into the emerging paradigm for patient-centered decisions about treatment (Table 4).
Table 4. Strategies to Improve Decision-Making Regarding Fertility Preservation in AYA Women With Cancer
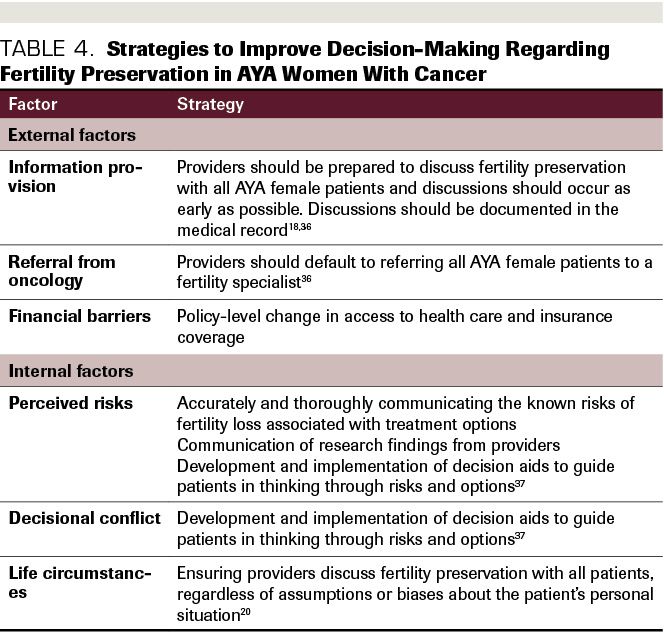
Another method for addressing these barriers to informed decision-making is the use of evidence-based decision aids, which can be videos, written information, or a product in another electronic format providing information about options, and may include coaching from a provider. Sometimes, these include values-clarification exercises, as well as information about potential priorities of care one should consider when making a treatment decision, including financial toxicity, logistics and timelines of treatment options, etc.
Decision aids are effective at improving patient involvement in medical decision-making and enabling them to be more knowledgeable and confident in their decisions. They may also improve outcomes such as quality of life and adherence to the chosen treatment.38,39 A recent systematic review of decision aids specifically designed to support fertility preservation decision-making found that their use decreased decisional conflict and increased knowledge about fertility planning, and users reported high overall satisfaction with them. However, only 2 such aids are currently available to AYA patients with cancer, and they are not tailored specifically to women.37 Building on the success of these aids, future work should develop and tailor them to improve psychosocial outcomes for female AYA cancer survivors.
The decision about whether to pursue fertility preservation is complicated for AYA women diagnosed with cancer. Despite tremendous strides made in recent years to not only define oncofertility as a field of study,11 but also to determine factors that make fertility preservation decisions difficult, little is known about the actual risk of fertility loss for AYA women with cancer. Current clinical guidelines are often based on data from pediatric cancer survivors due to the lack of longitudinal and epidemiological studies with diverse samples of this relatively new age group.40 These studies take time and are expensive to conduct, but have tremendous implications for fertility loss in AYA cancer survivors. Fertility loss due to cancer treatment depends on a number of factors in addition to the type of cancer and treatment, including individual differences in hormone levels before the cancer diagnosis and the variety of combinations of treatments and comorbidities in patients. The risk of fertility loss is currently difficult to predict, which in turn makes patient-provider communication and shared decision-making more difficult. Data indicating the risk of fertility loss by cancer type, treatment type, and other factors are necessary to determining treatment guidelines. In addition, as research progresses on fertility preservation options and improves outcomes, long-term health and psychosocial effects of undergoing, or not undergoing, fertility preservation treatment will be needed.
Additional unmet needs of AYA cancer survivors include high financial burden, negative impact on achievement regarding education and work, impaired relationships, and uncertainty about family planning, sexual functioning, and struggles with physical and mental health.21,33,41 Anxiety, self-blame, anger, and stress due to fertility loss also contribute to the difficulties experienced by this specific group. AYA women cancer survivors, in particular, suffer from negative psychosocial effects of cancer, including changes in physical appearance and functioning, emotional coping, and changes in social roles and in relationships, which exacerbate the already difficult psychological ramifications of fertility loss.42 Although the long-term impact of informed, shared decision-making on these outcomes has yet to be fully explored in AYA women cancer survivors, the psychological study of decision-making provides several opportunities for further inquiry into this area of research. This includes best practices for shared decision-making between patients and providers, coping styles and strategies, sense of control and self-efficacy in decision-making, values clarification, and decisional conflict and regret.8,43 Decision aids are an empirically validated way of incorporating shared decision-making. Currently, there are only 2 peer-reviewed decision aids regarding fertility preservation available to cancer patients, neither of which are tailored to AYA women.37 This could inform clinical conversations and survivorship needs, but also potential relationships between pretreatment individual differences and later outcomes.
Although more AYA women seem to be engaging in discussions with their oncologists about the risk of fertility loss and fertility preservation options, likely due to the publication of clinical guidelines from societies such as ASCO,36 a significant number are not being provided with the information they desire and are not making fully informed treatment decisions.20,22 Despite advances in fertility preservation methods for women, male AYA patients are still more likely to receive information about fertility preservation options and more likely to utilize them.44,45 The impact of gender discordance in the patient-provider dyad may also make discussing fertility preservation options more difficult for some patients.20 Providers and care teams make more immediate impact by providing information to AYA women, referring them to fertility preservation consultations, and starting the discussion early and openly. Prior findings suggest that these actions should be taken as early in the treatment planning phase as possible to allow patients to digest the risk of fertility loss and have as much time as possible to make informed decisions.18 Fully informed decisions that occur as early as possible may also decrease
anticipated regret and increase perceived control over uncertainty for patients who may otherwise choose to achieve this by pursuing fertility preservation, possibly delaying treatment to their detriment in the process. Future studies of shared decision-making about fertility preservation should include measures of psychosocial constructs such as these to elucidate the importance of perceived involvement in decision-making. In addition, further study of the impact of treatment delays due to fertility preservation is needed in diverse settings.9 As patients progress to survivorship, regardless of their fertility preservation treatment decision, care teams should continue to monitor fertility outcomes of AYA women and provide them information regarding family planning to protect against further distress, especially as the extent of damage to fertility due to treatment is often uncertain.19,46
Fertility is a top priority for AYA women undergoing cancer treatment with long term impacts on psychosocial and emotional wellbeing. Further study of the fertility preservation decision-making process will aid in ensuring patients are making fully informed decisions, as well as in intervention development to lessen decisional conflict before treatment and alleviate regret after treatment and into long-term survivorship. For providers to fully inform their patients of infertility risk, clinical and longitudinal data regarding fertility outcomes of AYA women cancer survivors are needed.
When deciding on a treatment plan, providers should discuss risks to female fertility and sexual function concurrently with the likelihood of remission and survival, with prompt referral to fertility specialists regardless of the initial perceived threat and priority of fertility. By adequately addressing fertility with the same urgency and precision as the underlying cancer through shared decision-making tools, we will continue to move toward a more patient-centered model of total cancer care.
REFERENCES
