Surgical Management of Hepatic Breast Cancer Metastases
Tremendous gains have been made regarding the treatment of breastcancer. The combination of chemotherapy, radiation therapy, and surgeryhave vastly improved patient course. Hepatic manifestations ofmetastatic breast cancer are extremely difficult to treat. Traditionally,chemotherapy and hormonal treatment of hepatic metastases of breastcarcinoma have not significantly improved survival. For patients withbreast cancer metastases isolated to the liver, operative treatment isincreasingly being used to prolong life and disease-free intervals. Thisarticle reviews the use of surgery for treatment of isolated breast cancermetastases to the liver.
Tremendous gains have been made regarding the treatment of breast cancer. The combination of chemotherapy, radiation therapy, and surgery have vastly improved patient course. Hepatic manifestations of metastatic breast cancer are extremely difficult to treat. Traditionally, chemotherapy and hormonal treatment of hepatic metastases of breast carcinoma have not significantly improved survival. For patients with breast cancer metastases isolated to the liver, operative treatment is increasingly being used to prolong life and disease-free intervals. This article reviews the use of surgery for treatment of isolated breast cancer metastases to the liver.
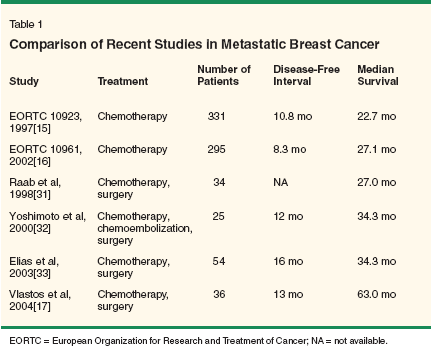
Tremendous gains have been made regarding the treatment of breast cancer. The combination of chemotherapy, radiation therapy, and surgery have vastly improved the patient's course. However, when breast cancer spreads, survival is limited. Of patients who develop breast cancer metastases, more than half have hepatic involvement as a component of their systemic disease.[1] Very few (approximately 5%) have disease located solely within the liver.[2,3] This group of patients with limited metastatic disease is the focus of this article. Hepatic manifestations of metastatic breast cancer are extremely difficult to treat. These tumor populations rarely maintain estrogen and progesterone receptor positivity, rendering hormonal treatment of no utility.[4] In these patients, standard application of regional and systemic chemotherapy has not improved outcome, with median survivals ranging from 1 to 4 months.[2,3,5] Much of this can be explained when considering breast cancer as a systemic disease. In many epithelial tumors such as breast carcinoma, immunocytochemical and molecular techniques have shown that occult tumor cells may circulate in the peripheral blood and find haven in the bone marrow. This can occur even in the absence of clinically apparent nodal or distant disease in up to 40% of patients.[6,7] Models have predicted that a mature tumor may seed upwards of 100,000 cells into the circulation daily, leading to more frequent clinically apparent metastases and worse survival.[8-11] Thus, when clinically or radiographically evident breast cancer is identified in the liver, it is a manifestation of systemic disease that probably includes undetectable metastases throughout the body. Rather than cure, cytoreduction strategies to prolong disease-free interval and overall survival are potential goals. Novel therapies such as chemoembolization and yttrium-90 radioembolization have failed to significantly improve long-term survival in other than anecdotal cases.[12,13] Medical therapies are of limited benefit in treating hepatic metastases of breast carcinoma. The latest European Organization for Research and Treatment of Cancer (EORTC) study to address breast cancer patients with only hepatic metastases was published in 2003.[14] This and earlier reports highlighted two European trials of first-line therapies: EORTC 10923, in which doxorubicin was compared to paclitaxel, and EORTC 10961, in which doxorubicin and cyclophosphamide were evaluated against doxorubicin and paclitaxel (Table 1).[14-16] In trial 10923, the median follow-up was 90.5 months; in trial 10961, it was 56.6 months. The median survival of patients with isolated hepatic metastases in EORTC 10961 was 22.7 months compared to 27.1 months in EORTC 10923. These were both significantly longer than in patients who suffered from extrahepatic disease. Time to progression of disease was 10.2 months in protocol 10923 and 8.3 months in protocol 10961. Overwhelmingly, patients had recurrences in the liver (96% in 10923, 60% in 10961).
Surgical Resection of Hepatic Breast Cancer Metastases With the acceptance of liverdirected medical, radiologic, and surgical treatment of colorectal cancer metastases, more attention has been generated in treating other tumors using these methods. Since 1988, interest in the survival of patients after hepatic resection of breast cancer metastases has resulted in approximately one publication per year. Five-year survival rates have ranged from 0% to 61%, with median survivals between 15 and 47 months.[17-24] Early US Trials
Although early reports of hepatic resection in this setting showed improvements over purely medical therapy, results were still not encouraging, with 5-year survivals for patients with breast cancer metastases to the liver ranging from 0%[25] to 11%.[19] These early reports identified extrahepatic disease and tumor bulk to predict worse overall survival.[26] A larger analysis of noncolorectal nonneuroendocrine hepatic metastases, of which 29 of 141 were from breast cancer, showed the disease-free interval from surgical breast cancer diagnosis and margin status of the hepatic resection to be significant in predicting survival.[27] In a large retrospective series from 1991, Wolf et al reported the results in 143 patients who underwent liver resection for metastatic disease.[28] Pathologies included adrenal, carcinoid, breast, sarcoma, melanoma, pancreatic, gastric, and Wilms tumors, with 5-year survival rates ranging from 0% for pancreatic metastases to 43% for carcinoid tumors. Of these, 14 had breast cancer metastases. The 5-year survival rate was only 7%, and median survival was 20 months. Early European Trials
The early European experience was similar. Berney and colleagues reported a 10% 5-year survival rate in their patients undergoing surgical resection of liver metastases from breast primaries.[ 29] Disease-free survival was 0% at 5 years. Analysis of their entire metastatic experience found a single hepatic metastatic focus and curative intent of operation to be the only indicators of good prognosis. As nonsurgical treatments of breast cancer improved, better results were seen in those with liver metastases. A 1998 report of 35 patients undergoing hepatectomy for isolated metastatic breast cancer accrued between 1984 and 1996 showed promising results.[ 30] With a mean of 4.5 liver lesions per patient, 66% underwent at least a three-segment hepatectomy. With little perioperative morbidity and mortality, 5-year overall survival was 35% after hepatectomy. Similarly, Raab et al reported results after 35 hepatectomies in 34 patients accrued from 1983 and 1994.[31] In this report, the median age was 47 years (range: 36-63 years), and the median interval between primary operation and liver resection was 27.3 months; 67% of patients initially presented with stage I or II disease. Approximately 70% of the lesions were over 3 cm in diameter and 41% of patients had more than a single lesion, with 33% having bilobar disease. Overall, median survival was 27 months. In the 30 cases where R0 resection was possible, median survival was 41.5 months compared to only 5 months for those treated with an R1 or R2 resection. Finally, in patients with a prior local recurrence at the breast operation site, median survival was 16%. Complications included seven bile leaks, two cases of postoperative bleeding, and a single infection. Operative mortality was 3%. Early Japanese Trials
The Japanese experience is similar to that seen in Europe. Yoshimoto reported 25 cases of hepatectomy for breast cancer metastases to the liver.[ 32] The liver was the first known site of recurrence in 19 of the 25 patients. Each patient except one who was 20 weeks pregnant was treated preoperatively with chemotherapy. In addition to preoperative computed tomography (CT) and magnetic resonance image (MRI) scans, intraoperative ultrasound was used to assess the abdomen for unresectable disease and any hepatic lesions not seen prior to exploratory laparotomy. Using this regimen, 17 patients (68%) were found to have disease clinically isolated to the liver. Of the original 25 patients, 14 had a single metastatic lesion, 3 had 2 lesions, 4 had 3 lesions, and 4 had 4 or more lesions. Tumor diameters ranged from 1.3 to 7.0 cm (mean: 4.1 cm). All resections were R0. In addition to hepatectomy, most patients underwent lymph node sampling in the porta hepatic and paraaortic chains, with 42% revealing breast cancer metastases.[32] Median survival was 34.3 months, with overall 2- and 5-year survival rates of 71% and 27%, respectively. Of note, 12 of the 25 patients undergoing hepatectomy had liver recurrences between 2 and 27 months after surgery. The remaining 13 patients had no hepatic recurrences after an average of 35 months (range: 6-132 months). On statistical analysis, only the presence of lymph node metastases in the abdomen predicted greater risk to survival. More Recent Investigations
Another larger study was published in 2003 by Elias et al.[33] In that study, 54 patients underwent hepatectomy for liver metastases from breast cancer during a 15-year period. Patient selection criteria included the lack of extrahepatic disease, World Health Organization performance status of 0 or 1, lesions amenable to safe, nonrisky resection, and an objective response of the lesions to chemotherapy or hormonal therapy. Of 65 women who initially underwent exploratory laparotomy, 11 were found to have disease not confined to the liver. These 11 patients survived a mean of 9.6 months. Intraoperative ultrasound discovered additional liver metastases in 20 patients (47.6%). The 54 patients included in the study received a median of five courses of preoperative doxorubicin-based chemotherapy that was repeated after the operation.[33] Sixteen patients were treated with a combination of systemic and hepatic artery infusion chemotherapy postoperatively. Of 32 hormone-receptor-positive patients, 30 (94%) received tamoxifen. A mean of 4.0 breast lesions were treated per patient. Hilar lymph nodes were positive in 17 patients. R0 resection was accomplished in 44 patients (81.4%). Median survival was 34.3 months, with overall survival 50% at 3 years and 34% at 5 years. Disease-free survival was 42% at 3 years and 22% at 5 years. On statistical analysis, only positive hormonal status was associated with an improvement in outcome (P = .03). Those with positive hormone status survived a median of 44 months, compared to only 19 months in those with negative estrogen and progesterone receptor status. The largest North American experience in this setting was published by Vlastos et al.[17] To undergo resection, patients had to be free of extrahepatic disease both preoperatively and with intraoperative ultrasound. A combination of hepatectomy with 1-cm margins and radiofrequency ablation with 1-cm margins was used to treat the liver disease. Of 36 patients initially seen for surgical resection of presumed isolated breast cancer liver metastases, 31 underwent operative therapy. The median age was 46 years (range: 31-70 years) with 55% of patients initially presenting with stage I or II disease. Only 6 (19%) underwent breast conservation; 81% were treated with preoperative chemotherapy, and 45% received endocrine therapy. At the time of surgery, median tumor size was 2.5 cm, and 81% were metastases from invasive ductal carcinoma. Hepatic disease developed at a median of 22 months after initial diagnosis (range: 0-144 months).[17] Of the resected lesions, only 58% were estrogen receptor-positive and 35% were progesterone receptor-positive. With no operative mortality, a median survival of 63 months was achieved. Actuarial 2- and 5-year survival rates were 86% and 61%, respectively. Median disease-free survival was 13 months. Several variables were studied to determine which, if any, independently affect survival. None, including age, initial stage, diseasefree interval, chemotherapy response, and receptor status, were found to significantly affect outcome. Preoperative Work-up From the series listed above, both for chemotherapeutic and surgical treatments of hepatic breast cancer metastases, it is evident that patients with disease isolated to the liver fare much better. Surgical resections in patients with extrahepatic disease fail to increase survival enough to make the morbidity and mortality of the operation worthwhile. In addition to CT scans of the abdomen and chest preoperatively, positron-emission tomography with F-18-fluorodeoxyglucose positron-emission tomography (FDGPET) has shown promise in identifying hepatic metastases.[34] Intraoperative Ultrasound and Radiofrequency Ablation Intraoperative ultrasound, first used in hepatobiliary surgery in 1963 by Knight,[35] is an important adjunct when used during laparoscopy and laparotomy. Prior to laparotomy, diagnostic laparoscopy with intraoperative ultrasound has shown promise in identifying metastatic foci not seen on prior studies, precluding unnecessary laparotomy.[36] In comparison to transabdominal ultrasound and CT, intraoperative ultrasound can image lesions as small as 3 mm with sensitivities and specificities over 90%.[37] One in five patients has hepatic tumors found on intraoperative ultrasound that were not seen preoperatively on CT, and one in three has additional lesions seen compared to laparoscopy.[38,39] Using intraoperative ultrasound, the entire liver can be visualized, and the relationship of any lesions to important vascular and biliary structures is readily attained. With this information, lesions less amenable to ablation, including those near major vascular and biliary structures, can be identified and resected. However, due to imaging limitations, intraoperative ultrasound may not identify small lesions close to Glisson's capsule. Radiofrequency ablation can be performed percutaneously, laparoscopically, or as an open surgical procedure. In small isolated lesions or in patients who present substantial perioperative risks, percutaneous ablation by CT guidance can be done on an outpatient basis. For lesions on the anterior or inferior surfaces of the liver that can be visualized with laparoscopic ultrasound, laparoscopic radiofrequency ablation provides another alternative. For deeper lesions, those near major structures, or those requiring mobilization of the liver for access, open radiofrequency ablation remains the best option. Conclusions Multiple small studies have confirmed that hepatectomy for isolated hepatic breast cancer metastases is safe and offers a survival advantage over standard chemotherapy regimens. Liver resection and/or ablation, although cytoreductive in nature, should not be done when extrahepatic disease is present or when complete tumor resection or destruction is not possible. As systemic therapies improve, more patients will likely present with breast cancer metastases isolated to the liver only. These patients should be carefully screened, evaluated, and counseled prior to surgical intervention. However, when possible, these patients should undergo surgery. Prospective, randomized trials of resection and ablation vs solely chemotherapeutic protocols must be performed to show the long-term affect of these treatments and their utility to patients. After exhaustive evaluation for extrahepatic disease, a group of highly selected patients will be candidates for a locoregional approach to their hepatic metastases. Although there are limited series for comparison, the ideal patient is one who has had a relatively long disease-free interval, small-sized lesions, significant response to chemotherapy, positive hormone-receptor status, and, most importantly, lack of extrahepatic metastatic disease. Unless contraindicated, the surgically cured patient should be assumed to have systemic disease and, therefore, treated with adjuvant cytotoxic or antihormonal therapy.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jardines L, Callans LS, Torosian MG: Recurrent breast cancer: Presentation, diagnosis, and treatment. Semin Oncol 20:538-547, 1993.
2. Hoe AL, Royle GT, Taylor I: Breast liver metastases-incidence, diagnosis and outcome. J R Soc Med 84:714-716, 1991.
3. Patanaphan V, Salazar OM, Risco R: Breast cancer: Metastatic patterns and their prognosis. South Med J 81:1109-1112, 1988.
4. Samaan NA, Buzdar AU, Aldinger KA, et al: Estrogen receptor: A prognostic factor in breast cancer. Cancer 47:554-560, 1981.
5. O’Reilly SM, Richards MA, Rubens RD: Liver metastases from breast cancer: The relationship between clinical, biochemical, and pathological features and survival. Eur J Cancer 26:544-547, 1990.
6. Pantel K, Muller V, Auer M, et al: Detection and clinical implications of early systemic tumor cell dissemination in breast cancer. Clin Cancer Res 9:6326-6334, 2003.
7. Diel IJ, Kaufmann M, Costa SD, et al: Micrometastatic breast cancer cells in bone marrow at primary surgery: Prognostic value in comparison with nodal status. J Natl Cancer Inst 88:1652-1658, 1996.
8. Liotta LA, Kleinerman J, Saidel GM: Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res 34:997-1004, 1974.
9. Butler TP, Gullino PM: Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res 35:512-516, 1975.
10. Chang YS, di Tomaso E, McDonald DM, et al: Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A 97:14608- 14613, 2000.
11. Cote RJ, Rosen PP, Lesser ML, et al: Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol 9:1749- 1756, 1991.
12. Giroux MF, Baum FA, Soulen MC: Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol 15:289- 291, 2004.
13. Rubin D, Nutting C, Jones B: Metastatic breast cancer in a 54-year-old woman: Integrative treatment with yttrium-90 radioembolization. Integr Cancer Ther 3:262- 267, 2004.
14. Atalay G, Biganzoli L, Renard F, et al: Clinical outcome of breast cancer patients with liver metastases alone in the anthracyclinetaxane era: A retrospective analysis of two prospective, randomized metastatic breast cancer trial. Eur J Cancer 39:2439-2449, 2003.
15. Awada A, Paridaens R, Bruning P, et al: Doxorubicin or Taxol as first-line chemotherapy for metastatic breast cancer (MBC): Results of an EORTC-IDBBC/ECSG randomized trial with crossover (EORTC 10923). Breast Cancer Res Treat 46:23a, 1997.
16. Biganzoli L, Cufer T, Bruning P, et al: Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: EORTC 10961. J Clin Oncol 20:3114-3121, 2002.
17. Vlastos G, Smith DL, Singletary SE, et al: Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol 11:869-874, 2004.
18. Pocard M, Pouillart P, Asselain B, et al: Hepatic resection for breast cancer metastases: Results and prognosis (65 cases). Ann Chir 126:413-420, 2001.
19. Stehlin JS, Hafstrom L, Greeff PJ: Experience with infusion and resection in cancer of the liver. Surg Gynecol Obstet 138:855-863, 1974.
20. Elias D, Lasser P, Spielmann M, et al: Surgical and chemotherapeutic treatment of hepatic metastases from carcinoma of the breast. Surg Gynecol Obstet 172:461-464, 1991.
21. Yoshimoto M, Sugitani I, Iwase T, et al: Therapeutic efficacy of hepatectomy in the treatment of hepatic metastases from breast cancer. Nippon Geka Gakkai Zasshi 96:174- 179, 1995.
22. Raab R, Bussbaum KT, Werner U, et al: Liver metastases in breast carcinoma: Results of partial liver resection. Chirurg 67:234-237, 1996.
23. Siefert JK, Weigel TF, Gonner U, et al: Liver resection for breast cancer metastases. Hepatogastroenterology 46:2935-2940, 1999.
24. Maksan SM, Lehnert T, Bastert G, et al: Curative liver resection for metastatic breast cancer. Eur J Surg Oncol 26:209-212, 2000.
25. Foster JH, Lundy J: Liver metastasis. Curr Probl Surg 18:157-202, 1981.
26. Stehlin JS, Ipolyi PD, Greeff, et al: 1Treatment of cancer of the liver. Ann Surg 208:23- 35, 1988.
27. Weitz J, Blumgart LH, Fong Y, et al: Partial hepatectomy for metastases from noncolorectal, non-neuroendocrine carcinoma. Ann Surg 241:269-276, 2005.
28. Wolf RF, Goodnight JE, Drag DE, et al: Results of resection and proposed guidelines for patient selection in instances of noncolorectal hepatic metastases. Surg Gynecol Obstet 173:454-460, 1991.
29. Berney T, Mentha G, Roth AD, et al: Results of surgical resection of liver metastases from non-colorectal primaries. Br J Surg 85:1423-1427, 1998.
30. Elias D, Cavalcanti de Albuquirque A, Eggenspieler P, et al: Resection of liver metastases from a noncolorectal primary: Indications and results based on 147 monocentric patients. J Am Coll Surg 187:487-493, 1998.
31. Raab R, Nussbaum KT, Behrend M, et al: Liver metastases of breast cancer: Results of liver resection. Anticancer Res 18:2231-2234, 1998.
32. Yoshimoto M, Tada T, Saito M, et al: Surgical treatment of hepatic metastases from breast cancer. Br Cancer Res Treat 59:177-184, 2000.
33. Elias D, Maisonnette F, Druet-Cabanac M, et al: An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg 185:158-164, 2003.
34. Donckier V, Van Laethem JL, Goldman S, et al: [F-18] Fluorodeoxyglucose positron emission tomography as a tool for early recognition of incomplete tumor destruction after radiofrequency ablation of liver metastases. J Surg Oncol 84:215-223, 2003.
35. Knight PR, Newell JA: Operative use of ultrasonics in cholelithiasis. Lancet 1:1023- 1025, 1963.
36. Chung MH, Wood TF, Tsioulias GJ, et al: Laparoscopic radiofrequency ablation of unresectable hepatic malignancies. A phase 2 trial. Surg Endosc 15:1020-1026, 2001.
37. Machi J, Oishi AJ, Furumoto NL, et al: Intraoperative ultrasound. Surg Clin N Am 84:1085-1011, 2004.
38. Foroutani A, Garland Am, Berber E, et al: Laparoscopic ultrasound vs. triphasic computed tomography for detecting liver tumors. Arch Surg 135:933-938, 2000.
39. John TG, Greig JD, Crosbie JL, et al: Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg 220:711-719, 1994.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.