Targeted Therapy in Advanced Urothelial Carcinoma
This review focuses on targeted therapies related to these pathways of interest for the treatment of advanced urothelial carcinoma, describing the evidence to support further investigation of these approaches.
Urothelial carcinoma (UC) is a common and deadly cancer in the United States. While molecularly targeted therapies have been integrated into the standard-of-care management of other solid tumors in recent years, the use of targeted therapy in UC has lagged behind. Accordingly, the management of advanced disease, along with outcomes, has remained largely unchanged for the past 2 decades. Despite the lack of new agents in the clinic, preclinical and early clinical studies have demonstrated that numerous potentially “targetable” molecular pathways exist, including the epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), human epidermal growth factor receptor 2 (HER2/neu), and insulin-like growth factor 1 receptor (IGF1R) pathways. This review focuses on targeted therapies related to these pathways of interest for the treatment of advanced UC, describing the evidence to support further investigation of these approaches. Notably, the identification and validation of new agents will only occur through accrual to urothelial cancer trials designed to answer these questions, which will require the support of the entire urologic community.
Bladder cancer was the fourth most common and eighth most deadly cancer in males in the United States in 2012, with an estimated 55,600 new cases and 17,910 deaths. In females, an estimated 14,580 new cases and 10,510 deaths occurred in the same year.[1] The most common histologic type of bladder cancer, urothelial carcinoma (UC), accounts for more than 90% of cases. At diagnosis, 30% of cases are muscle-invasive, conferring a significant risk of locally advanced or distant metastatic disease. UC is a chemotherapy-sensitive tumor, with chemotherapy response rates around 50% in trials of modern, combination, platinum-based chemotherapy; however, UC often recurs, with a median survival of 14 to 15 months despite chemotherapy in the metastatic setting. Historically, the standard of care in the locally advanced or metastatic setting was MVAC (methotrexate, vinblastine, Adriamycin [doxorubicin], cisplatin) chemotherapy. However, in 2000, a phase III trial by von der Maase et al demonstrated a survival, response rates, and time to progression equal to MVAC using gemcitabine-cisplatin (GC), with an improved toxicity profile compared to MVAC.[2] Since that time, GC has become a new standard-of-care systemic therapy for locally advanced or metastatic UC. Attempts to improve response rates beyond those seen with GC and MVAC have included the addition of a taxane to GC and the administration of MVAC in a dose-dense fashion. Phase II studies have shown activity with a gemcitabine, platinum, and taxane combination that is promising compared to GC alone.[3] A phase III study comparing GC-paclitaxel to GC alone showed better response rates and a 3-month overall survival benefit with GC-paclitaxel, although the latter did not quite reach statistical significance.[4] In addition, a phase III trial of dose-dense MVAC (HD-MVAC) vs conventional MVAC showed a better overall response rate and progression-free survival for HD-MVAC but equivalent median overall survival for the two regimens.[5]
Despite these efforts to identify new uses for and combinations of traditional cytotoxic chemotherapy in UC, survival has improved very little in the last 2 decades. Furthermore, although new-generation biologic/targeted agents have been integrated into the treatment of other cancer types, these therapies have no current use in standard therapy for advanced UC. Notably, UC has a rich array of potentially “druggable” targets, including the vascular endothelial growth factor (VEGF), epidermal growth factor receptors 1 and 2 (EGFR and HER2/neu), and insulin-like growth factor (IGF) pathways, all of which are implicated in the pathogenesis of UC. It must be acknowledged that the failure to integrate biologic agents into the routine care of UC patients is not the result of negative results from large, phase III studies, but of the inability to initiate and complete phase III studies of these agents in UC.
EGFR
The human epidermal growth factor receptor (HER) family is composed of four receptor tyrosine kinases: HER1 (Erb-B1 or EGFR), HER2 (Erb-B2 or Neu), HER3 (Erb-B3), and HER4 (Erb-B4). These receptors exist in their inactivated state as cell surface monomers; however, when bound by their appropriate ligand, they undergo homo- or heterodimerization, leading to downstream signaling via a number of pathways that ultimately results in cellular proliferation and survival. EGFR and HER2/neu in particular have been implicated in the pathogenesis of invasive UC.
TABLE 1

EGFR-Targeted Agents Under Investigation for Urothelial Cancer
The EGFR receptor is a plasma membrane glycoprotein that dimerizes after ligand activation. This leads to autophosphorylation, resulting in downstream signaling that contributes to cell proliferation, survival, angiogenesis, and metastasis. EGFR is implicated in many cancers, including UC, in which EGFR is overexpressed compared with normal tissue. More than 50% of human UCs overexpress EGFR, and the level of expression correlates with stage, grade, and survival.[6,7] EGFR is naturally expressed in the epithelial layers, and is expressed in the basal cells of the urothelial mucosa, as well as in all layers of the urothelium.[8] The ligand for this receptor, EGF, has been found to induce or stimulate the proliferation of UC cells.[9,10] Dinney et al established an orthotopic model of UC by implanting 253J malignant urothelial cells into the bladder wall of nude mice; the cell line was then altered to overexpress EGFR, which resulted in increased metastasis, supporting the contribution of EGFR to malignant progression. In addition, treatment of 253J cells with an EGFR inhibitor, cetuximab (Erbitux), resulted in a dose-dependent decrease in cell proliferation.[11] Subsequent therapy with cetuximab in an in vivo model has resulted in regression of local tumors and decreased metastasis.[12] In addition to the above-mentioned studies, other in vitro work has supported inhibition of EGFR-expressing cancer cells by additional anti-EGFR monoclonal antibodies.
Various inhibitors of EGFR have been developed, either as monoclonal antibodies (cetuximab, panitumumab [Vectibix]) or as small-molecule receptor inhibitors (erlotinib [Tarceva], gefitinib [Iressa]). Urothelial tumors with a more “epithelial”-as opposed to “mesenchymal”-phenotype have higher EGFR expression and appear to be quite sensitive to EGFR inhibitors. Thus, the use of EGFR blockade with erlotinib is being explored in early-stage, non−muscle-invasive UC as a means of preventing the potential transition to the mesenchymal phenotype that carries a higher likelihood of muscle invasion and metastatic spread (NCT 00749892). However, preclinical work by Bue et al has also shown strong membrane staining for EGFR in metastatic deposits of UC, making EGFR blockade a rational strategy in the metastatic setting as well.[13]
Gefitinib is an orally active selective EGFR tyrosine kinase inhibitor with in vitro activity in UC.[14] The Southwest Oncology Group (SWOG) study 0031 evaluated the role of single-agent gefitinib as second-line treatment for metastatic UC.[15] The study employed a Simon two-stage design and evaluated 6-month progression-free survival. Thirty-one patients were enrolled; there was one partial response, two patients had stable disease (6%), and 81% of the patients had progressed by the first evaluation. The median overall survival was 3 months. The two-stage design allowed for progression to the second step if ≥ 9/30 patients were progression-free at 6 months. Because the majority of patients had progressed by the first radiographic evaluation, the study was stopped after the first step. The most commonly reported toxicities were fatigue and rash. Blockade of EGFR by single-agent gefitinib in this setting is thus not recommended based on these negative results. Of note, no correlation was seen between expression of EGFR and response.
Additional evaluation of gefitinib in the metastatic setting in combination with chemotherapeutic agents was carried out in the Cancer and Leukemia Group B (CALGB) trial 90102.[16] This phase II trial evaluated cisplatin, 70 mg/m2, with gemcitabine, 1000 mg/m2, on days 1 and 8, plus gefitinib, 500 mg daily, for a 21-day cycle in patients with untreated metastatic disease. Out of 54 patients assessed, there were 23 objective responses (overall response rate, 42.6%) and a median overall survival of 15.1 months. The median time to progression was 7.4 months. Although the combination appeared active, the results were not significantly better than historical data for GC alone. Additional clinical studies of gefitinib include an ongoing phase II trial of docetaxel compared to docetaxel plus gefitinib as maintenance therapy for patients already treated with first-line chemotherapy. The primary endpoint of this trial (NCT00479089) is progression-free survival at 9 months from the start of consolidation.
Erlotinib is a reversible oral kinase inhibitor of EGFR approved for the treatment of non−small-cell lung cancer. This drug was also evaluated in the setting of neoadjuvant therapy for UC.[17] This phase II trial evaluated erlotinib dosed at 150 mg daily as neoadjuvant treatment for muscle-invasive UC. Twenty patients were enrolled to receive 4 weeks of preoperative therapy, with subsequent surgical resection and potential maintenance therapy for patients with organ-confined disease. The primary endpoint was the rate of pathologic complete response. At the time of cystectomy, 25% of patients had no residual disease, 35% had down-staging to less than pT2 disease, and 75% had organ-confined disease. Twelve patients had organ-confined disease at surgery and continued on adjuvant erlotinib for a mean of 29 weeks. At 24.8 months of follow-up, 10 patients were disease-free and 5 had received cytotoxic chemotherapy for node-positive disease. The most common adverse event was a rash, seen in 15 of the 20 patients, grade ≥ 3 in 4 of those patients. The treatment was otherwise well tolerated and appeared to have some activity, but this neoadjuvant approach has not been explored further in phase III trials. Another phase II trial (NCT 00749892) is currently evaluating a similar role for erlotinib in the neoadjuvant setting; this trial may offer additional information on the merits of future exploration of this agent, or of erlotinib in combination with other agents in this setting.
TABLE 2

HER2/
neu
-Targeted Agents Under Investigation for Urothelial Cancer
Cetuximab is a human/murine chimeric monoclonal antibody against EGFR that is approved for treatment of colon cancer and head and neck cancer.[18] Preclinical data suggesting activity led to phase I and II clinical trials in patients with UC.[19] A randomized, open-label, noncomparative phase II study evaluated cetuximab with and without paclitaxel in metastatic UC that had progressed after a previous line of chemotherapy.[20] Thirty-nine patients were evaluated, with 11 in the single-agent arm and 28 in the combination arm. The single-agent arm was closed for futility when 9 of 11 patients had progressed by the first disease evaluation at 8 weeks. The combination arm accrued 28 patients who were treated with 4-week cycles of cetuximab, 250 mg/m2, given with paclitaxel, 80 mg/m2, per week. The overall response rate was 25%, with three complete responses and four partial responses. Median progression-free survival was 16.4 weeks, and median overall survival was 42 weeks. Seventeen of the 28 patients had visceral metastasis, with a median progression-free survival of 16.1 weeks. The most common treatment-related adverse events were rash, fatigue, and hypomagnesemia. This study concluded that single-agent cetuximab is inactive in advanced UC, but the responses seen in the combination arm suggest a signal in combination with paclitaxel. The combination arm met the study endpoint of improved progression-free survival compared with historical controls; however, further studies have not been planned at this time. This combination may be interesting in a comparative trial. Results of a randomized phase II trial of GC given with or without cetuximab in patients with unresectable, locally recurrent, or metastatic UC were recently reported.[21] Patients received gemcitabine, 1000 mg/m2, on days 1, 8, and 15, with cisplatin, 70 mg/m2, on day 1. Those in the cetuximab-containing arm also received cetuximab, 500 mg/m2, on days 1 and 15 of a 28-day cycle. Patients were then continued on monotherapy with cetuximab after 4 to 6 cycles. Thrombotic complications prompted a protocol alteration to lower the gemcitabine dose in the combination arm. Of the eligible patients, 28 were randomly assigned to the standard therapy arm and 56 to the cetuximab-containing arm. Progression-free survival and median survival were not improved in the experimental arm. In addition, the combination arm contained more adverse events, including more thromboembolism (6.9% vs 18.6% in the cetuximab arm), hyponatremia (3.5% vs 10.2%), rash (0% vs 28.8%), and fatigue-and two deaths. Thus cetuximab has not clearly improved upon current therapies and does appear to carry significant toxicity.
HER2/neu
TABLE 3
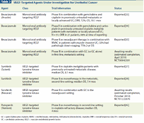
VEGF-Targeted Agents Under Investigation for Urothelial Cancer
HER2/neu has become a therapeutic target in multiple solid tumors. Agents targeting HER2/neu, in particular trastuzumab (Herceptin), are part of standard-of-care therapy in HER2/neu-positive breast cancer in both the metastatic and adjuvant settings, and they also have a role in HER2/neu-expressing gastric cancer. In UC, HER2/neu gene amplification and protein overexpression have been investigated in a number of studies, with variable findings. In one series of 245 patients with UC, 45% expressed HER2/neu, which correlated with survival both when present alone or when co-expressed with either EGFR or HER3.[22] In another series, Jimenez et al evaluated 80 consecutive patients with muscle-invasive UC and found that 28% expressed HER2/neu in their primary tumor, while 53% expressed HER2/neu in locoregional lymph node metastases.[23] Median survival for those with HER2/neu-positive tumors was 33 months, compared with 50 months for those with HER2/neu-negative tumors, but this did not reach statistical significance. Kruger et al evaluated 138 consecutive patients undergoing radical cystectomy for muscle-invasive UC and found 41% expressed HER2/neu; that expression was positively correlated with tumor grade and negatively correlated with disease-related survival.[24] Another series found that, despite a high frequency of HER2/neu expression in high-grade muscle-invasive UC (71%), expression was actually associated with improved survival in the setting of paclitaxel chemotherapy.[25] Despite these mixed results with respect to the prognostic implications of HER2/neu in UC, it is clear that HER2/neu is frequently expressed in UC, and successful targeting of this pathway in other solid tumors makes it an attractive target in this setting.
Several studies have investigated the role of anti-HER2/neu therapies in UC. A small 2005 series of six patients treated with trastuzumab, either alone or in combination with platinum- and/or taxane-based chemotherapy, showed survival ranging from 8 to 22 months in the metastatic setting.[26] A subsequent phase II trial in 2007 investigated the safety and efficacy of trastuzumab in combination with gemcitabine, carboplatin, and paclitaxel in 44 patients with HER2/neu-expressing advanced UC.[27] Overall response rate was 70%; median time to progression, 9.3 months; and median overall survival, 14 months-results that are similar to historical results with current standard-of-care therapy in this setting. In this study, patients with HER2/neu-positive tumors had a greater median number of metastatic sites and more frequent visceral metastasis than their counterparts with HER2/neu-negative tumors. Notably, there was a suggestion of increased cardiac toxicity in this trial, with 23% of participants experiencing a cardiac adverse event (grade 1−3). To fully elucidate the role of trastuzumab in HER2/neu-positive advanced UC, a randomized trial is needed.
Lapatinib (Tykerb) is an oral tyrosine kinase inhibitor (TKI) with dual specificity for EGFR and HER2/neu that has been shown to have in vitro activity against UC cell lines. McHugh et al showed that lapatinib acts synergistically, in both a cytostatic and cytotoxic sense, with combination chemotherapy.[28] Interestingly, this observation held in UC cell lines with both high and low HER2/neu expression, which underscores the complexity of predicting tumor cell response to lapatinib, as was highlighted in a prior study in which tissue type, along with both EGFR and HER2/neu expression, were important determinants of lapatinib response.[29] Despite the complexity of predicting tumor cell response to lapatinib, the suggestion of activity in UC cell lines in vitro has led to early-phase clinical trials incorporating lapatinib into UC therapy. In a single-arm, open-label, multi-center phase II study, Wulfing et al investigated lapatinib monotherapy in patients with metastatic UC that had progressed on first-line platinum-based chemotherapy.[30] Approximately one-third of participants achieved stable disease, but overall response rate, the study’s predetermined response criterion, was only 1.7%. Median time to progression was 8.6 weeks, with a median overall survival of 17.9 weeks. Clinical benefit (overall response rate + stable disease) was associated with EGFR overexpression (P = .02), and median overall survival was improved in patients whose tumors overexpressed EGFR and/or HER2/neu (P = .0001). This suggests that further trials specifically studying participants with HER2/neu and/or EGFR overexpression are warranted, and continued investigation of predictive biomarkers in this setting is of paramount importance. Further ongoing trials of lapatinib in UC include a phase II/III randomized study of maintenance lapatinib vs placebo in patients with metastatic UC overexpressing EGFR and/or HER2/neu with at least stable disease after first-line chemotherapy (NCT00949455). An additional phase I study is investigating GC with lapatinib in patients with previously untreated, HER2/neu-overexpressing metastatic or locally advanced UC (NCT00623064).
A novel effort is also assessing the effectiveness of autologous cellular immunotherapy against HER2/neu in the adjuvant UC setting. Sipuleucel-T (Provenge) is an approved agent for metastatic, castration-resistant prostate cancer that employs this same form of immunotherapy to target prostate acid phosphatase rather than HER2. In the UC study, eligible patients with residual disease at the time of cystectomy are tested for HER2/neu, and those with HER2/neu tissue expression are randomly assigned to receive either no therapy or HER2/neu-directed autologous cellular immunotherapy (NCT01353222).
VEGFR
A number of studies have suggested a relationship between angiogenesis (specifically as measured via microvessel density), tumor behavior, and prognosis in UC.[31] In vitro data have also shown that the inhibition of angiogenesis can decrease tumor growth, metastasis, and neovascularization.[32] These relationships have provided the impetus for targeting angiogenesis in the treatment of UC. The vascular endothelial growth factor (VEGF) and its receptor (VEGFR) are clinically useful therapeutic targets in other solid organ malignancies, including colorectal cancer, non−small-cell lung cancer, renal cell carcinoma, and glioblastoma, and their relevance in UC is being investigated in clinical trials. A phase II trial by the Hoosier Oncology Group investigated the activity of cisplatin, gemcitabine, and bevacizumab (Avastin; a humanized monoclonal antibody against VEGF) in patients with previously untreated metastatic or locally advanced and inoperable UC.[33] The overall response rate was encouraging at 72%. The median progression-free survival was 8.2 months, and the median overall survival was 19.1 months, both of which compared favorably with historical outcomes with standard-of-care therapy in this setting. Notably, 21% of patients experienced venous thromboembolism (VTE), 7% experienced hemorrhage, and there were three treatment-related deaths (central nervous system hemorrhage, aortic dissection, sudden cardiac death) in the study. A significantly higher rate of VTE (39% vs 8%) was observed with higher-dose gemcitabine (1250 mg/m2) than with lower-dose gemcitabine (1000 mg/m2). Another phase II trial from Memorial Sloan-Kettering Cancer Center assessed the activity of carboplatin, gemcitabine, and bevacizumab in cisplatin-ineligible patients with metastatic UC.[34] The results, presented at the 2011 ASCO Genitourinary Symposium, included a response rate of 42% and median overall survival of 13.9 months. Bevacizumab has also been tested in the neoadjuvant setting, with a phase II trial at MD Anderson Cancer Center evaluating the activity of dose-dense MVAC with bevacizumab in high-risk patients with muscle-invasive UC. Nearly half of the patients had pathologic down-staging (< pT2) with therapy, and 2-year overall survival was 75%.[35] To more definitively elucidate the role of bevacizumab in the metastatic setting, an ongoing multicenter phase III trial (CALGB 90601) is comparing GC to GC plus bevacizumab (NCT00941331).
In addition to bevacizumab, a number of TKIs that target the VEGF pathway have been developed, and early evaluation of these in advanced bladder cancer is ongoing. Sunitinib (Sutent), which targets the VEGF and platelet-derived growth factor (PDGF) pathways, among other pathways, has shown in vitro and xenograft in vivo activity against UC cell lines, where a synergistic effect with cisplatin has been observed.[36] Despite these results, clinical trials of sunitinib in UC have yielded overall disappointing results. A phase II trial of sunitinib as first-line therapy for metastatic UC in cisplatin-ineligible patients demonstrated a median overall survival of 8.1 months, less than that seen with carboplatin-based chemotherapy in this setting.[37] Sunitinib has also led to disappointing results when combined with platinum-based chemotherapy in the neoadjuvant setting.[38] Perhaps the most promising results have occurred using sunitinib as monotherapy in the second-line, metastatic setting, where no standard of care exists; a median overall survival of 7.1 months was seen in a phase II trial.[39] Sorafenib (Nexavar) is another multitargeted small-molecule TKI that targets the VEGF and other pathways. Results in UC have been poor, with no demonstrated efficacy in phase II trials, either as monotherapy in the first- or second-line setting, or when combined with GC in the first-line, advanced setting, where both toxicity and survival were equal between the GC-alone arm and the GC-plus-sorafenib arm.[40-42] A study of sorafenib added to GC in the neoadjuvant setting is ongoing (NCT01222676). A third small-molecule TKI with activity against the VEGF pathway, pazopanib (Votrient), has demonstrated in vitro activity against UC cell lines, particularly in combination with docetaxel.[43] Pazopanib has thus been investigated in early-phase clinical trials in UC. Two phase II trials of pazopanib in the second-line, metastatic setting have yielded conflicting results. Pili et al found no objective responses in the first 16 patients treated in this setting, and their trial was closed early.[44] Necchi et al recently reported results of a trial of second-line pazopanib in cisplatin-refractory disease, in which 76% of the 41 studied patients derived clinical benefit (17% partial response, 59% stable disease). Median overall survival in this study was 4 months, suggesting that further study of pazopanib in UC is warranted.[45]
Vandetanib (Caprelsa) is a TKI that targets both EGFR and VEGFR. It was recently assessed in a randomized phase II study of docetaxel with or without vandetanib in patients with advanced UC with progression after platinum-based chemotherapy.[46] The primary objective was an improvement in progression-free survival. The study found no difference in the overall survival or response rates between the two arms. Progression-free survival was slightly improved in the combination arm, but this did not reach significance. In vitro studies of vandetanib in UC suggest that higher concentrations of the agent induce G1 arrest, with increased levels of cyclin-dependent kinase p27, potentially reducing the effects of cytotoxic chemotherapy in combination therapy and suggesting the need to consider timing and scheduling in the integration of these agents with cytotoxic chemotherapy.[47]
IGF1R
The insulin-like growth factor 1 receptor (IGF1R) is a trans-membrane receptor tyrosine kinase that has been implicated in the development and progression of several human cancers, including UC.[48] Activation of the heterodimeric receptor ultimately results in decreased apoptosis, increased cell growth, and increased mobility, leading to tumor growth and metastasis. Given its importance in cancer and influence over many cell growth and survival pathways, IGF1R has emerged as a target of interest in UC. IGF1R and insulin-like growth factor 2 (IGF2) are overexpressed in UC cells,[49] contributing to the invasion, metastatic potential, and chemotherapy sensitivity of cancer cells.[50,51] This more invasive phenotype is induced by the secretion of matrix metalloproteinases through AKT- and mitogen-activated protein kinase (MAPK)-dependent activation of paxillin.[52] By stimulating T24 and 5637 UC cells with IGF1, cells were increasingly able to migrate and invade a three-dimensional matrix.[52] Through siRNA silencing of IGF1R, the ability of cells to migrate was severely inhibited. A recent study examined the role of IGF1R in promoting the transition to invasive disease through analysis of IGF1R levels in cancer and normal bladder tissue and found that IGF1R expression by immunohistochemistry increased with grade in UC compared with benign control tissue.[53]
While we have little clinical experience using the blockade of IGF1R as a treatment for UC, there are numerous IGF1R inhibitors in clinical development. Early trials of monoclonal antibodies yielded disappointing results in phase II and III trials; however, new small-molecule agents that are in development-and that have improved specificity and shorter half-lives-may prove more promising.[54] Thus, based on preclinical evidence, there is a rationale for further exploration in the clinical arena.
Conclusion
In summary, UC is a common and deadly cancer in which conventional cytotoxic chemotherapy is of transient utility for many patients, and the median overall survival in the advanced disease setting has changed little in the last 2 decades. While efforts to incorporate targeted therapies into UC management are evolving, they lag behind those in other common solid tumors. Further investigation of targeted therapies is needed to improve survival of patients with advanced UC. Going forward, the entire urologic oncology community will need to work together to design and complete practice-changing phase III studies in this area. The lack of data supporting new therapies has been a result of poor study accrual; thus, the genitourinary oncology community should take as its charge a focus on completing these trials. Currently, the large Intergroup effort through the CALGB-90601 trial exemplifies the sort of focused effort that is needed.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29.
2. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068-77.
3. Hussain M, Vaishampayan U, Du W, et al. Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J Clin Oncol. 2001;19:2527-33.
4. Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30: 1107-13.
5. Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19:2638-46.
6. Chow NH, Liu HS, Lee EI, et al. Significance of urinary epidermal growth factor and its receptor expression in human bladder cancer. Anticancer Res. 1997;17:1293-6.
7. Nguyen PL, Swanson PE, Jaszcz W, et al. Expression of epidermal growth factor receptor in invasive transitional cell carcinoma of the urinary bladder. A multivariate survival analysis. Am J Clin Pathol. 1994;
101:166-76.
8. Andrawis R, Contrino J, Lindquist R, et al. Interleukin-8 expression and human bladder cancer: in situ and in vitro expression of IL-8 by human bladder cancer cells. J Urol. 1997;157:28.
9. Neal DE, Mellon K. Epidermal growth factor receptor and bladder cancer: a review. Urol Int. 1992;48:
365-71.
10. Messing EM. Growth factors and bladder cancer: clinical implications of the interactions between growth factors and their urothelial receptors. Sem Surg Oncol. 1992;8:285-92.
11. Bellmunt J, Hussain M, Dinney CP. Novel approaches with targeted therapies in bladder cancer. Therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit Rev Oncol/Hematol. 2003;46(Suppl):S85-104.
12. Mothe I, Ballotti R, Tartare S, et al. Cross talk among tyrosine kinase receptors in PC12 cells: desensitization of mitogenic epidermal growth factor receptors by the neurotrophic factors, nerve growth factor and basic fibroblast growth factor. Mol Biol Cell. 1993;4:737-46.
13. Bue P, Wester K, Sjostrom A, et al. Expression of epidermal growth factor receptor in urinary bladder cancer metastases. Int J Cancer. 1998;76:189-93.
14. Shrader M, Pino MS, Brown G, et al. Molecular correlates of gefitinib responsiveness in human bladder cancer cells. Mol Cancer Ther. 2007;6:277-85.
15. Petrylak DP, Tangen CM, Van Veldhuizen PJ, Jr, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int. 2010;105:317-21.
16. Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol. 2009;20:1074-9.
17. Pruthi RS, Nielsen M, Heathcote S, et al. A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: clinical and pathological results. BJU Int. 2010;106:349-54.
18. Goldstein NI, Prewett M, Zuklys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311-8.
19. Inoue K, Slaton JW, Perrotte P, et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res. 2000;6:4874-84.
20. Wong YN, Litwin S, Vaughn D, et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol. 2012;30:3545-51.
21. Grivas P, Agarwal N, Siefker-Radtke A, et al. Randomized phase II trial of gemcitabine/cisplatin (GC) with or without cetuximab (CET) in patients (pts) with advanced urothelial carcinoma (UC). J Clin Oncol. 2012;30(suppl):Abstr 4506.
22. Chow NH, Chan SH, Tzai TS, et al. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:1957-62.
23. Jimenez RE, Hussain M, Bianco FJ, Jr, et al. HER2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res. 2001;7:2440-7.
24. Kruger S, Weitsch G, Buttner H, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications. Int J Cancer. 2002 ;102:514-8.
25. Gandour-Edwards R, Lara PN, Jr, Folkins AK, et al. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer. 2002;95:1009-15.
26. Peyromaure M, Scotte F, Amsellem-Ouazana D, et al. Trastuzumab (Herceptin) in metastatic transitional cell carcinoma of the urinary tract: report on six patients. Eur Urol. 2005;48:771-5; discussion 5-8.
27. Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol. 2007;25:2218-24.
28. McHugh LA, Sayan AE, Mejlvang J, et al. Lapatinib, a dual inhibitor of ErbB-1/-2 receptors, enhances effects of combination chemotherapy in bladder cancer cells. Int J Oncol. 2009;34:1155-63.
29. Rusnak DW, Alligood KJ, Mullin RJ, et al. Assessment of epidermal growth factor receptor (EGFR, ErbB1) and HER2 (ErbB2) protein expression levels and response to lapatinib (Tykerb, GW572016) in an expanded panel of human normal and tumour cell lines. Cell Prolif. 2007;40:580-94.
30. Wulfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115:2881-90.
31. Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Nat Cancer Inst. 1995;87:1603-12.
32. Inoue K, Chikazawa M, Fukata S, et al. Frequent administration of angiogenesis inhibitor TNP-470 (AGM-1470) at an optimal biological dose inhibits tumor growth and metastasis of metastatic human transitional cell carcinoma in the urinary bladder. Clin Cancer Res. 2002;8:2389-98.
33. Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol. 2011;29:1525-30.
34. Balar AV, Milowsky MI, Apolo AB, et al. Phase II trial of gemcitabine, carboplatin, and bevacizumab in chemotherapy-naive patients (pts) with advanced/metastatic urothelial carcinoma (UC). J Clin Oncol. 2011;29:(suppl 7):Abstr 248.
35. Siefker-Radtke AO, Kamat AM, Corn PG, et al. Neoadjuvant chemotherapy with DD-MVAC and bevacizumab in high-risk urothelial cancer: results from a phase II trial at the MD Anderson Cancer Center. J Clin Oncol. 2012;30(18 suppl):Abstr 261.
36. Sonpavde G, Jian W, Liu H, et al. Sunitinib malate is active against human urothelial carcinoma and enhances the activity of cisplatin in a preclinical model. Urol Oncol. 2009;27:391-9.
37. Bellmunt J, Gonzalez-Larriba JL, Prior C, et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol. 2011;22:2646-53.
38. Lerner SP, Powles T, Hahn NM, et al. A phase II trial of neoadjuvant cisplatin (C), gemcitabine (G), and sunitinib (S) in muscle-invasive urothelial carcinoma (miUC): results from Hoosier Oncology Group GU07-123 trial. J Clin Oncol. 2011;29(7 suppl):Abstr e15173.
39. Gallagher DJ, Milowsky MI, Gerst SR, et al. Phase II study of sunitinib in patients with metastatic urothelial cancer. J Clin Oncol. 2010;28:1373-9.
40. Sridhar SS, Winquist E, Eisen A, et al. A phase II trial of sorafenib in first-line metastatic urothelial cancer: a study of the PMH Phase II Consortium. Invest New Drug. 2011;29:1045-9.
41. Dreicer R, Li H, Stein M, et al. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115:4090-5.
42. Krege S, Rexer H, vom Dorp P, et al. Gemcitabine and cisplatin with or without sorafenib in urothelial carcinoma (AUO-AB 31/05). J Clin Oncol. 2010;28(15 suppl):Abstr 4574.
43. Li Y, Yang X, Su LJ, Flaig TW. Pazopanib synergizes with docetaxel in the treatment of bladder cancer cells. Urology. 2011;78:233 e7-13.
44. Pili R, Qin R, Flynn PJ, et al. MC0553: A phase II safety and efficacy study with the VEGF receptor tyrosine kinase inhibitor pazopanib in patients with metastatic urothelial cancer. J Clin Oncol. 2011;29(7 suppl):Abstr 259.
45. Necchi A, Zaffaroni N, Mariani L, et al. Biomarker analysis and final results of INT70/09 phase II proof-of-concept study of pazopanib (PZP) in refractory urothelial cancer (UC). Proceedings of the 2012 American Association of Cancer Research Annual Meeting; Chicago, IL: March 31–April 4, 2012. Abstr LB-433.
46. Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30:
507-12.
47. Flaig TW, Su LJ, McCoach C, et al. Dual epidermal growth factor receptor and vascular endothelial growth factor receptor inhibition with vandetanib sensitizes bladder cancer cells to cisplatin in a dose- and sequence-dependent manner. BJU Int. 2009;103:1729-37.
48. Yuen JS, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as a treatment for cancer. Expert Opin Ther Targets. 2008;12:589-603.
49. Cooper MJ, Fischer M, Komitowski D, et al. Developmentally imprinted genes as markers for bladder tumor progression. J Urol. 1996;155:2120-7.
50. Dunn SE, Hardman RA, Kari FW, Barrett JC. Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of HBL100 human breast cancer cells by inhibition of apoptosis induced by diverse anticancer drugs. Cancer Res. 1997;57:2687-93.
51. Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339-53.
52. Metalli D, Lovat F, Tripodi F, et al. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol. 2010;176:2997-3006.
53. Rochester MA, Patel N, Turney BW, et al. The type 1 insulin-like growth factor receptor is over-expressed in bladder cancer. BJU Int. 2007;100:1396-401
54. Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem. 2009;1:1153-71.