Thromboembolic Complications of Malignancy: Part 1
Thromboembolism affects many patients with solid tumors and clonalhematologic malignancies. Pathogenetic mechanisms include inflammatory-and tissue factor-mediated coagulation, natural anticoagulantdeficiencies, fibrinolytic alterations, hyperviscosity, and activationof platelets, endothelial cells, and leukocytes. High rates of venousthromboembolism (VTE) occur with advanced pancreatic, breast, ovarian,germ cell, lung, prostate, and central nervous system cancers.Hodgkin disease, non-Hodgkin's lymphoma, myeloma, paroxysmalnocturnal hemoglobinuria, and certain leukemias also predispose tovenous thromboembolism. Arterial and venous events occur with polycythemiavera and essential thrombocythemia. Central venous cathetersand prothrombotic antitumor regimens augment the risk in somepatients. Part 1 of this two-part article addresses pathophysiology, clinicalpresentations, and risk of malignancy-associated thrombosis. Part 2,which will appear in next month's issue, covers prophylaxis and treatmentof these thromboembolic complications.
Thromboembolism affects many patients with solid tumors and clonal hematologic malignancies. Pathogenetic mechanisms include inflammatory- and tissue factor-mediated coagulation, natural anticoagulant deficiencies, fibrinolytic alterations, hyperviscosity, and activation of platelets, endothelial cells, and leukocytes. High rates of venous thromboembolism (VTE) occur with advanced pancreatic, breast, ovarian, germ cell, lung, prostate, and central nervous system cancers. Hodgkin disease, non-Hodgkin's lymphoma, myeloma, paroxysmal nocturnal hemoglobinuria, and certain leukemias also predispose to venous thromboembolism. Arterial and venous events occur with polycythemia vera and essential thrombocythemia. Central venous catheters and prothrombotic antitumor regimens augment the risk in some patients. Part 1 of this two-part article addresses pathophysiology, clinical presentations, and risk of malignancy-associated thrombosis. Part 2, which will appear in next month's issue, covers prophylaxis and treatment of these thromboembolic complications.
Over half of all adult cases of venous thromboembolism (VTE), including deep venous thrombosis and pulmonary embolism, occur in the setting of acquired, situational, hereditary, pharmacologic, and/or iatrogenic prothrombotic risk factors.[1,2] Recent or active cancer is one of the most powerful independent and additive acquired hypercoagulable conditions, approximating or exceeding the attributable risks associated with advanced age, acute infectious illness, or a prior history of VTE.[2] The annual incidence of VTE among all cancer patients has been estimated to be 0.5%, with 10- to 20- fold higher rates among those with advanced ovarian, breast, pancreatic, and lung cancer, brain tumors, myeloproliferative disorders (ie, polycythemia vera and essential thrombocythemia), myeloma, and those treated with thrombogenic antitumor regimens.[3-5] Thromboembolic complications of malignancy significantly affect quality of life and may profoundly compromise overall management and outcome. Compared to VTE in individuals without cancer, malignancy-associated deep vein thrombosis and pulmonary embolism are associated with longer hospitalization times, a twofold higher 28-day mortality rate, a threefold higher rate of readmission and/or death at 6 months, and two- to fourfold greater risks of subsequent bleeding (on warfarin anticoagulation) and/or recurrent thrombosis (with or without continued anticoagulation).[5-7] Moreover, concurrent cancer increases the likelihood of perioperative venous and arterial thrombotic complications by two- to threefold.[3] The 1-year survival rate of patients with cancer-associated VTE is onethird lower than the survival rate of cancer patients who remain thrombosis- free (12% vs 36%), reflecting, in part, the more advanced stage of disease among thrombotic patients.[8] These thromboembolic complications translate into higher health-care expenditures[ 7] and spotlight the need for more selective, aggressive, and cost-effective interventions in this high-risk population. Part 1 of this two-part review summarizes pathophysiology, clinical presentation, and risk of malignancyassociated thrombosis. In part 2, which will appear in next month's issue, we will explore the management of thromboembolic complications in cancer patients, including the specifics of prophylaxis and treatment, cost considerations, and future perspectives. Pathophysiology of Malignancy- Associated ThrombosisVenous vs Arterial Vascular Events
The prothrombotic mechanisms that affect the vascular systems in patients with malignancies mirror those that are important in individuals without cancer. In general, venous thrombosis results from abnormalities that affect blood flow, the integrity of the venous endothelium and/or the hemostatic balance of activated procoagulants, natural anticoagulants, fibrinolytic mediators and, in some cases, platelets. Most acquired and congenital prothrombotic conditions alter those hemostatic mechanisms and, thereby, predominantly lead to deep venous thrombosis and pulmonary embolism.[1,2,9] Pharmacologic agents that inhibit one or more steps in the coagulation cascade, including unfractionated heparin, low-molecular-weight heparin, warfarin, or more specific inhibitors of factor Xa (eg, fondaparinux [Arixtra]) or thrombin (eg, argatroban, lepirudin [Refludan], bivalirudin [Angiomax]) are required to prevent and treat VTE. Given the minor role of platelets in this process, drugs that reversibly or irreversibly inhibit platelet function (eg, aspirin, clopidogrel [Plavix], ticlopidine, dipyridamole) are not effective for VTE prophylaxis or therapy. Arterial vascular events generally occur as a result of underlying atherosclerotic disease that ultimately leads to acute platelet-fibrin thrombotic occlusion and/or downstream emboli. In some cases, arterial emboli originate in the heart (eg, from a left atrial thrombus or valvular vegetations) or, paradoxically, from VTEs that traverse an intracardiac shunt into the arterial circulation. Antiplatelet agents decrease the chance of primary and secondary atherothrombotic complications and they may partially reduce the risk of cardioembolic events. Acute arterial thrombosis is treated with systemic anticoagulants and, in some cases, thrombolytic agents (eg, recombinant tissue-type plasminogen activator [tPA]). Malignancy-associated thromboembolic complications usually occur in the venous system; however, some conditions predispose to arterial thromboemboli. Patients with polycythemia vera, essential thrombocythemia, and disorders complicated by disseminated intravascular coagulation (DIC), heparin-induced thrombocytopenia with thrombosis, or nonbacterial thrombotic endocarditis (also known as "marantic" endocarditis), are at increased risk of arterial events.[3,4,10] In addition, a recent retrospective cohort study observed an overall 1.5% incidence of arterial thromboemboli among 66,106 patients hospitalized for malignancy and neutropenia.[11] Interestingly, onehalf of those arterial events involved patients with leukemia and lymphoma, and the annual frequency more than doubled from 1995 to 2002. Prothrombotic Mechanisms and Altered Laboratory Values
The prothrombotic mediators and mechanisms implicated in malignancy- associated hypercoagulability are summarized in Table 1. The presence and severity of these abnormalities relate, in part, to the underlying histology and stage of disease, associated comorbid conditions, and antitumor treatments.[12] Importantly, some of these mechanisms may also participate in tumor progression by facilitating microinvasion and metastasis. In addition, neoangiogenesis may be stimulated through tissue factor-induced upregulation of vascular endothelial growth factor (VEGF). The hypothetical link between malignancy-associated hypercoagulability and tumor biology has been supported by clinical data showing a decreased incidence of subsequent primary cancer diagnosis among patients with primary VTE who received longer-duration warfarin therapy[13] and improved survival among a subset of cancer patients who received low-molecular-weight heparin for VTE prophylaxis.[14] Alterations in routine hemostatic laboratory markers may reflect direct effects of cancer-associated prothrombotic factors, activation of downstream mediators, and/or the products of the malignant cells themselves (Table 1).[12,15,16] Among the most common examples of causal association are acute promyelocytic leukemia and advanced gastrointestinal (GI) ad- enocarcinomas, both of which are associated with high rates of thrombosis and laboratory markers of DIC (ie, elevated fibrin degradation products and/ or D-dimer, increased prothrombin time, increased activated partial thromboplastin time, increased thrombin time, decreased fibrinogen, and/or decreased platelet count).
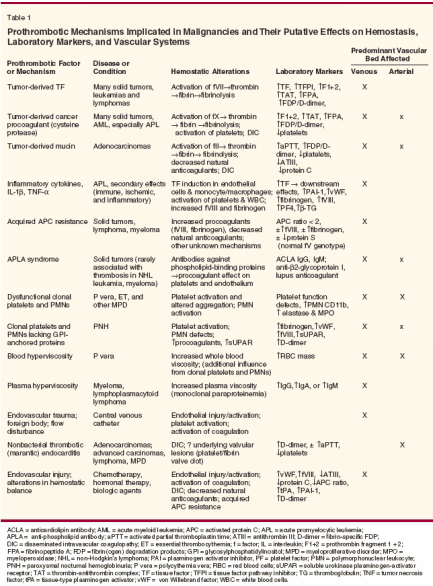
By comparison, patients with polycythemia vera and essential thrombocythemia are at increased risk of venous and arterial thrombosis, and endogenous platelet aggregation defects are frequently demonstrable by in vitro assays. The presence or absence of qualitative platelet defects, however, does not correlate with the thrombotic risk observed in patients with polycythemia vera or essential thrombocythemia.[4] In most patients with malignancies, routine hemostatic assays are normal or only nonspecifically altered, and these findings do not predict clinical complications.[15] More specialized or research-based assays for cellular-derived and plasma coagulation factors and hemostatic activity detect abnormalities in subsets of cancer patients (Table 1).[15-17] Increased levels of tissue factor (the major activator of factor VII), platelet factor 4 (a marker of platelet activation), procoagulants and/or markers of thrombin/fibrin generation (ie, prothrombin fragment 1 + 2 [F1+2], thrombin-antithrombin complex, fibrinopeptide A, and tPA), have been observed in patients with adenocarcinomas (particularly of the GI or genitourinary [GU] tract) and advanced solid tumors, and after acute thrombotic complications or recurrent thrombosis on anticoagulant therapy.[ 17] Additional abnormalities include antiphospholipid antibodies, primary fibrinolysis, and decreased activities of natural anticoagulants, including protein S, antithrombin III (ATIII), protein C, and/or the activated protein C (APC) complex.[16,17] The question of whether specific laboratory alterations might identify high-risk individuals who would benefit from prophylactic anticoagulation has been addressed in various studies. One prospective myeloma treatment trial observed an increased risk of VTE among patients with acquired APC resistance.[18] Other recent studies have assessed coagulation markers in cancer patients at the time of VTE and compared those with markers in cancer patients without VTE. These trials have found correlations between acute VTE and acquired APC resistance or levels of thrombin-antithrombin III complex (TAT), F1+2, tPA, protein C activity, and/or von Willebrand factor antigen.[16] Recurrent VTE has been associated with Ddimer and TAT levels.[17] Before these hemostatic markers can be used to guide thromboprophylaxis in the clinic, confirmatory evidence is needed from well-designed prospective trials among patients with similar disease types and treatment courses. Clinical Presentations of Malignancy-Associated ThrombosisOccult Malignancy in Patients With VTE
Thrombosis may be the first clinical sign of an underlying malignancy. Roughly 7% to 10% of adults with idiopathic VTE (ie, not predisposed by an identifiable preexisting risk factor), will be diagnosed with cancer at the time of presentation or within the next 6 to 24 months.[19,20] This is especially true for older individuals. Because the prevalence of cancer is low in younger adults, the relative risk of an associated malignancy is actually highest among those under age 60.[20] The malignancies most likely to be discovered include non-Hodgkin's lymphoma (NHL) and carcinomas of the pancreas, ovary, liver, brain, GI tract, lung, breast, and GU system. Approximately 10% to 25% of patients with unrecognized polycythemia vera or essential thrombocythemia will be diagnosed at the time of an arterial or venous thromboembolic event and/or will have a history of previous thrombosis. Similarly, up to 20% of patients with occult paroxysmal nocturnal hemoglobinuria (PNH), an acquired hematopoietic clonal disorder, will present with venous or, less commonly, arterial thrombosis.[21] Of clinical impor- tance, the thrombotic complications of PNH, polycythemia vera, or essential thrombocythemia may involve unusual anatomic sites, such as the hepatic veins (Budd-Chiari syndrome), mesenteric, portal, and splenic veins, and cerebral sinuses. Retrospective studies and small prospective trials have suggested that extensive screening for occult malignancy in adults with VTE is not beneficial or cost-effective.[19,20] Either routine testing was felt to be sufficient to uncover cancer-related abnormalities in most patients and/or the prognosis would not have been affected by earlier detection. However, a more recent randomized screening trial[22] and a prospective cohort study[23] found that roughly half of preexisting cancers in patients with VTE are not detected by routine screening (ie, a complete physical examination, routine blood counts, blood chemistries, urine testing, and chest x-ray), but can be found with more extensive studies. Prostate-specific antigen, carcinoembryonic antigen, CA-125, alpha-fetoprotein, abdominopelvic imaging (by ultrasound and/or computed tomography), or additional imaging studies and endoscopy (as indicated) identified the occult cancer in 50% to 90% of cases. In addition, many of the malignancies found with extensive screening at the time of VTE were at earlier stages than the tumors diagnosed 8 to 11 months later among patients who had undergone initial routine testing, suggesting that some patients might benefit from extensive screening. No studies have yet determined whether extensive screening and earlier detection of occult malignancies in patients with VTE affect overall prognosis and survival. The one recent randomized clinical trial designed to address these questions could not complete accrual.[22] Thus, until evidence- based guidance is available, the clinician must be cognizant of important clinical and laboratory indicators to prompt additional evaluation (Table 2).[21-23] Based on recent studies, imaging of the abdomen, pelvis, and chest would be an efficient next step in the search for an occult solid tumor.

Thrombosis in Patients With Known Malignancies
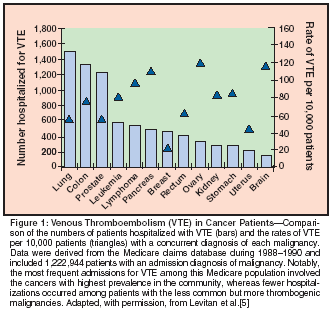
The risks of VTE among patients with known malignancies have been estimated by retrospective analyses of treatment cohorts, patient registries, and Medicare claims databases.[5,24,25] The highest incidence rates, ranging from 76 to 120 events per 10,000 patient admissions, occur among patients with ovarian, brain, pancreatic, gastric, renal, and colorectal cancers (Figure 1).[5] Increased incidence is also seen with lymphoma, leukemia, myeloma, liver, lung, prostate, gynecologic, and breast cancers. Some retrospective studies, but not others, have identified advanced tumor stage (compared to limited stage) and/or recent chemotherapy (compared to no therapy) as strong independent risk factors. From the clinician's perspective, hospitalizations for deep venous thrombosis or pulmonary embolism most frequently involve malignancies that are more prevalent in the community (such as lung, colon, prostate, and breast cancers, leukemia, and lymphoma) than malignancies that are most highly thrombogenic (Figure 1).[5]
Prospective treatment trials have provided additional important data regarding the incidence rates of thrombosis among patients with certain solid tumors. The 5-year rate of VTE in women with stage I/II breast cancer on no adjuvant treatment is roughly 0.2%, but is fourfold or 20-fold higher for women on tamoxifen or on chemotherapy plus tamoxifen, respectively. In addition, roughly 4% to 18% of women with advanced-stage breast cancer on chemotherapy suffer a thromboembolic event (reviewed in [3]). Similarly, VTE affects 11% of women during treatment for ovarian cancer,[ 26] 8% of men on chemotherapy for germ cell cancer (especially those with liver metastasis and receiving high-dose corticosteroids),[27] up to 26% of patients with malignant glioma (especially those with lowerextremity paresis, reviewed in [28]), 4% to 7% with lung cancer (especially adenocarcinoma and metastatic disease),[ 29,30] 2% to 25% with prostate cancer (particularly among those receiving estrogenic agents),[31] and 15% to 28% with pancreatic cancer (especially those with metastatic disease).[32] Among the hematologic malignancies, roughly 6% to 13% of patients with Hodgkin's disease or NHL develop VTE.[33,34] Many of these events are related to tumor-associated vessel compression, catheter-associated thrombosis, more advanced disease stage, and/or earlier course of treatment. Notably, deep venous thrombosis and/or pulmonary embolism develop in 60% of patients with central nervous system (CNS) lymphoma.[35] VTE occurs in 4.8% of patients following stem cell transplantation for hematologic malignancies, including 0.7% who develop arterial events.[36] Predisposing risks include indwelling central catheters, line infection, sepsis, and pulmonary disease. Recent treatment trials for myeloma have demonstrated VTE in 10% to 15% of patients, with up to 35% incidence rates among some cohorts on thalidomide (Thalomid)- containing regimens.[37] Polycythemia vera and essential thrombocythemia are associated with a 5% to 10% annual risk of thromboembolic events.[4,38] The majority of those events affect the arterial system (ie, stroke and myocardial infarction) and occur most commonly in older individuals (ie, age over 65 years) and/or among those with a prior thrombotic history. Thrombosis may also occur in patients with acute leukemias and concurrent disseminated intravascular coagulation, especially among those with acute promyelocytic leukemia. Roughly 3% to 38% of patients with acute lymphoblastic leukemia on asparaginase (Elspar)- and/or prednisone- containing regimens suffer deep venous thrombosis, CNS venous or arterial events, and catheter-related thrombosis.[39,40] PNH is associated with a 10-year risk of VTE ranging from 4% to 44%, with the highest risk among nonaplastic patients who circulate a high proportion of clonal PNH granulocytes.[21,41] Additional Prothrombotic Risks in Patients With MalignanciesAntitumor and Supportive Agents
Therapeutic agents may further increase the risks of thromboembolism in patients with malignancies. Chemotherapeutic drugs, hormones, and biologic agents might contribute to hypercoagulability by injuring or killing tumor cells, causing the release of prothrombotic mediators. Alternatively, they might directly activate or perturb endothelial cells and platelets, stimulate the release of tissue factor and/or procoagulants, interfere with natural anticoagulants, alter fibrinolysis, or trigger inflammatory pathways (Table 3).
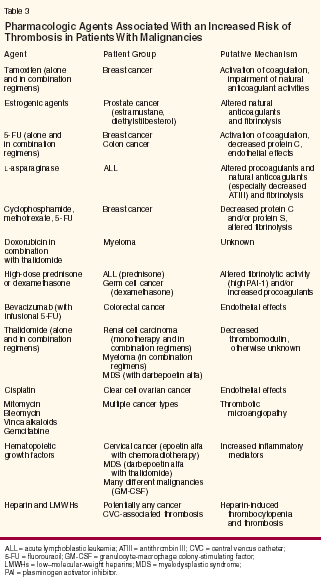
The magnitude and clinical consequences of a prothrombotic drug may vary according to the underlying disease and use of adjunctive measures. For example, thalidomide alone increases the thrombotic risk in patients with renal cell carcinoma[42] but not in patients with myeloma.[43] Moreover, thalidomide appears to be most thrombogenic when combined with other agents, including doxorubicin, dexamethasone, gemcitabine (Gemzar), fluorouracil (5-FU), or darbepoetin alfa (Aranesp).[37,44] Such observations have lead to the recommendation that prophylactic anticoagulation be used with multiagent regimens containing thalidomide.[37] The thrombogenic potential of bevacizumab (Avastin), an anti-VEGF monoclonal antibody, is also most apparent when combined with vasculopathic agents such as infusional 5-FU.[45] Similarly, epoetin alfa (Epogen, Procrit) alone does not, in general, increase the VTE risk in cancer patients; however, high rates of central venous catheter (CVC)-associated upper-extremity deep venous thrombosis and lower-extremity events occurred when epoetin alfa was added to concurrent chemotherapy and radiation for cervical cancer.[46] More recently, epoetin alfa-related thromboembolic events led to the early closure of a randomized trial assessing postsurgical chemoradiotherapy for rectal and gastric cancer.[47] The risks of drug-associated VTE may be predictable with some agents and unpredictable with others. Estrogenic compounds and selective estrogen receptor modulators (SERMs) are known to alter the activities of natural anticoagulants and fibrinolytic processes, and, therefore, they increase the risk of VTE in noncancer patients as well as in patients with breast and prostate cancer. Similarly, asparaginase causes a predictable decline in ATIII levels, thereby increasing the risk of peripheral and CNS thrombosis. These observations have led to the investigational use of prophylactic AT infusions for patients with acute lymphoblastic leukemia receiving asparaginase- containing regimens.[39] By contrast, treatment-induced thrombotic complications can occur as a result of an idiosyncratic drug reaction. Such is the case with heparininduced thrombocytopenia and thrombosis as well as with thrombotic microangiopathy associated with mitomycin, gemcitabine, bleomycin, or vinca alkaloids. A large, ongoing prospective trial is under way to characterize the clinical parameters that correlate with chemotherapy- associated thrombosis.[11] So far, preliminary analyses have identified a baseline platelet count > 337,000/μL, the primary site of cancer (especially lymphoma, GI, and lung cancer), an Eastern Cooperative Oncology Group (ECOG) performance status ≥ 2, and baseline use of erythropoietin or colony-stimulating factors as important independent risk factors.[11] Further observations and validation will hopefully yield a useful predictive model to guide thromboprophylaxis in high-risk patients. Central Venous Catheter-Related Risks
CVC-associated thrombotic complications range from the relatively frequent occurrence of intraluminal occlusion (13% to 93% incidence), to symptomatic deep venous thrombosis (4% to 41% incidence) and/or pulmonary embolism (3% to 25%), to septic thrombophlebitis (uncommon).[ 48] Asymptomatic deep venous thrombosis and pulmonary embolism occur more frequently (up to 66% and 15% to 50%, respectively); however, these progress to symptomatic thrombosis in a minority of cases. Therefore, the clinical relevance and the potential benefit of thromboprophylaxis remain questionable. Of note, recent prospective prophylactic intervention trials and preliminary results from a cohort study observed symptomatic CVC-related thrombosis in only 3.4% to 4.3% of cases (reviewed in [49]), suggesting that newer catheter materials, insertion methods, and/ or care techniques reduce the risk. Multiple factors have been implicated in CVC-induced deep venous thrombosis and pulmonary embolism (Table 4).[48] Congenital and acquired hypercoagulable states, including antiphospholipid antibodies, the factor V G1691A mutation (ie, factor V Leiden), factor II G20210A mutation, homozygous methylenetetrahydrofolate reductase variant, ATIII deficiency, and/or protein S deficiency, have been implicated in some pediatric studies,[50] but not others.[39] Among adults, only factor V Leiden has been strongly linked to CVC thrombosis in patients undergoing allogeneic bone marrow transplantation[51] and women with locally advanced or metastatic breast cancer undergoing infusional therapy with 5-FU.[52]

The subclavian vein is the most common site for both asymptomatic and clinically relevant CVC-associated deep venous thromboses. Significantly fewer events involve the superior vena cava, brachial, jugular, or axillary veins. Clinical signs include limb swelling, paresthesias, erythema, neck/facial swelling, headache, jaw pain, venous engorgement, and collateral vein formation. Roughly 15% to 35% of patients with symptomatic deep venous thrombosis will develop postphlebitic syndrome.[48] Surgical and Radiation Therapy-Related Risks
Among patients with malignancies, major surgical interventions are associated with two- to threefold higher rates of perioperative venous and arterial thrombotic events, compared to surgeries in noncancer patients.[3] Independent risks include neurosurgery for brain cancer, intrathoracic or abdominopelvic resections, and age > 60 years. High rates of perioperative thrombosis are observed with lymphomas that compress or obstruct vascular structures. Roughly threefourths of patients with uncontrolled erythrocytosis due to polycythemia vera develop postoperative thrombosis (or bleeding). This risk is reduced to ≤ 5% if the hematocrit is normalized preoperatively. Deep venous thrombosis, pulmonary embolism, and portal vein thrombosis affect roughly 10% of patients following splenectomy for hematologic malignancies, including myeloproliferative disorders, chronic lymphocytic leukemia, and NHL.[53] Patients with myeloproliferative disorders who develop postsplenectomy "rebound" thrombocytosis (ie, > 600,000/μL) are at greatest risk for these complications. Radiation therapy for cancers can induce vascular stenosis, occlusion, and accelerated atherosclerosis, thereby increasing the chance of subsequent venous or arterial thromboembolic events. Radiochemotherapy combinations have been implicated in epoetin alfa-associated thrombosis[46,47]; however, it is unknown whether radiation induces an independent or additive prothrombotic stimulus in these regimens. Carotid and vertebral artery thrombosis may occur after neck irradiation.[ 54] Lower-extremity deep venous thrombosis can develop as a complication of venous stenosis following pelvic irradiation (eg, for gynecologic malignancies).[55] Similarly, axillary and subclavian vein stenosis/ thrombosis have been reported after axillary irradiation (eg, for lymphoma or breast cancer). Acquired and Congenital Primary Hypercoagulable States
A number of studies have assessed the potential role of acquired and congenital hypercoagulable states in malignancy- associated thrombosis. Some have identified certain factors that contribute to CVC-induced events (see above). Isolated reports have shown an effect of factor V Leiden in patients with gastrointestinal carcinoma[56] and antiphospholipid antibodies with essential thrombocythemia[57] or solid tumors.[58] Other cohort studies have not found an increased prevalence of the common genetic disorders among thrombotic patients with essential thrombocythemia or polycythemia vera, glioma, gynecologic malignancies, breast cancer, and hepatocellular carcinoma. By comparison, two recent case-control studies have observed an apparent additive influence for the factor V Leiden mutation[ 59] and/or the factor II G20210A mutation [59,60] in venous thrombosis risk among patients with solid tumors and hematologic malignancies.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Heit J, O'Fallon M, Petterson T, et al: Relative impact of risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med 162:1245-1248, 2002.
2. Alikhan R, Cohen A, Combe S, et al: Risk factors for venous thromboembolism in hospitalized patients with acute medical illness. Arch Intern Med 164:963-968, 2004.
3. Lee A, Levine M: Venous thromboembolism and cancer: Risks and outcomes. Circulation 107:I17-I21, 2003.
4. Spivak JL, Barosi G, Tognoni G, et al: Chronic myeloproliferative disorders. Hematology (Am Soc Hematol Educ Program):200- 224, 2003.
5. Levitan N, Dowlati A, Remick S, et al: Rates of initial and recurrent thromboembolic disease amoung patients with malignancy versus those without malignancy: Risk analysis using Medicare claims data. Medicine 78:285- 291, 1999.
6. Prandoni P, Lensing AW, Piccioli A, et al: Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 100:3484-3488, 2002.
7. Elting L, Escalante C, Cooksley C, et al: Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med 164:1653-1661, 2004.
8. Sorensen H, Mellemkjaer L, Olsen J, et al: Prognosis of cancers associated with venous thromboembolism. N Engl J Med 343:1846- 1850, 2000.
9. Crowther M, Kelton J: Congenital thrombophilic states associated with venous thrombosis: A qualitative overview and proposed classification system. Ann Intern Med 138:128-134, 2003.
10. Cestari DM, Weine DM, Panageas KS, et al: Stroke in patients with cancer. Neurology 62:2025-2030, 2004.
11. Khorana AA, Francis CW, Culakova, et al: Thromboembolism in hospitalized neutropenic cancer patients (abstract 2199). Blood 104:84a, 2004.
12. Rickles R, Falanga A: Molecular basis for the relationship between thrombosis and cancer. Thromb Res 02:V215-V224, 2001.
13. Schulman S, Lindmarker P: Incidence of cancer after prophylaxis with warfarin against recurrent venous thromboembolism. N Engl J Med 342:1953-1958, 2000.
14. Kakkar A, Levine M, Kadziola Z, et al: Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol 22:1944-1948, 2004.
15. Johnson MJ, Walker ID, Sproule MW, et al: Abnormal coagulation and deep venous thrombosis in patients with advanced cancer. Clin Lab Haematol 21:51-54, 1999.
16. Goldenberg N, Kahn S, Solymoss S: Markers of coagulation and angiogenesis in cancer-associated venous thromboembolism. J Clin Oncol 21:4194-4199, 2003.
17. Sallah S, Husain A, Sigounas V, et al: Plasma coagulation markers in patients with solid tumors and venous thromboembolic disease receiving oral anticoagulation therapy. Clin Cancer Res 10:7238-7243, 2004.
18. Zangari M, Saghafifar F, Anaissie E, et al: Activated protein C resistance in the absence of factor V Leiden mutation is a common finding in multiple myeloma and is associated with an increased risk of thrombotic complications. Blood Coagul Fibrinolysis 13:187-192, 2002.
19. Sorensen H, Mellemkjaer L, Steffensen F, et al: The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med 338:1169-1173, 1998.
20. Murchison JT, Wylie L, Stockton DL: Excess risk of cancer in patients with primary venous thromboembolism: A national, population- based cohort study. Br J Cancer 91:92- 95, 2004.
21. Nishimura J, Kanakura Y, Ware R, et al: Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine 83:193-207, 2004.
22. Piccioli A, Lensing A, Prins M, et al: Extensive screening for occult malignant disease in idiopathic venous thromboembolism: A prospective randomized clinical trial. J Thromb Haemost 2:884-889, 2004.
23. Monreal M, Lensing A, Prins H, et al: Screening for occult cancer in patients with acute deep vein thrombosis or pulmonary embolism. Thromb Haemost 164:190-194, 2004.
24. Otten Hans-Martin MB, Mathijssen J, Cate H, et al: Symptomatic venous thromboembolism in cancer patients treated with chemotherapy. Arch Intern Med 164:190-194, 2004.
25. Sallah S, Wan J, Nguyen N: Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb Haemost 87:575-579, 2002.
26. von Tempelhoff GF, Dietrich M, Niemann F, et al: Blood coagulation and thrombosis is patients with ovarian malignancy. Thromb Haemost 77:456-461, 1997.
27. Weijl NI, Rutten MF, Zwinderman AH, et al: Thromboembolic events during chemotherapy for germ cell cancer: A cohort study and review of the literature. J Clin Oncol 18:2169-2178, 2000.
28. Marras L, Geerts W, Perry J: The risk of venous thromboembolism is increased throughout the course of malignant glioma. Cancer 89:640-646, 2000.
29. Blom JW, Osanto S, Rosendaal FR: The risk of a venous thrombotic event in lung cancer patients: Higher risk for adenocarcinoma than squamous cell carcinoma. Thromb Haemost 2:1760-1765, 2004.
30. Behrendt C, Ruiz R: Venous thromboembolism among patients with advanced lung cancer randomized to prinomastat or placebo, plus chemotherapy. Thromb Haemost 90:734-737, 2003.
31. Lubiniecki GM, Berlin JA, Weinstein RB, et al: Thromboembolic events with estramustine phosphate-based chemotherapy in patients with hormone-refractory prostate carcinoma. Cancer 101:2755-2759, 2004.
32. Khorana A, Fine R: Pancreatic cancer and thromboembolic disease. Lancet Oncol 5:655-663, 2004.
33. Seifter EJ, Young RC, Longo DL: Deep venous thrombosis during therapy for Hodgkin's disease. Cancer Treat Rep 69:1011- 1013, 1985.
34. Komrokji RS, Uppal NP, Khorana AA, et al: Venous thromboembolic events in patients with diffuse large B cell non-Hodgkin's lymphoma (abstract 4542). Blood 104:219b, 2004.
35. Goldschmidt N, Linetsky E, Shalom E, et al: High incidence of thromboembolism in patients with central nervous system lymphoma. Cancer 98:1239-1242, 2003.
36. Kuderer NM, Khorana AA, Friedberg JW: Incidence and risk factors for thromboembolism in patients with hematologic malignancies undergoing allogeneic and autologous stem cell transplantation (abstract 1141). Blood 104:323a, 2004.
37. Zangari M, Barlogie B, Anaissie E, et al: Deep vein thrombosis in patients with multiple myeloma treated with thalidomide and chemotherapy: Effects of prophylactic and therapeutic anticoagulation. Br J Haematol 126:715-721, 2004.
38. Landolfi R, Marchioli R, Kutti J, et al: Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 350:114-124, 2004.
39. Mitchell LG, Andrew M, Hanna K, et al: A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with l-asparaginase: Results of the prophylactic antithrombin replacement in kids with acute lymphoblastic leukemia treated with asparaginase (PARKAA) study. Cancer 97:508-516, 2003.
40. Athale U, Chan A: Thrombosis in children with acute lymphoblastic leukemia. Part I: Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res 111:125-131, 2003.
41. Hall C, Richards S, Hillmen P: Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 102:3587-3591, 2003.
42. Amato R: Thalidomide therapy for renal cell carcinoma. Crit Rev Oncol Hematol 46:S59-S65, 2003.
43. Singhal S, Mehta J, Desikan R, et al: Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341:1565- 1571, 1999.
44. Steurer M, Sudmeier I, Stauder R, et al: Thromboembolic events in patients with myelodysplastic syndrome receiving thalidomide in combination with darbepoietin-alpha. Br J Haematol 121:101-103, 2003.
45. Kabbinavar F, Hurwitz H, Fehrenbacher L, et al: Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60- 65, 2003.
46. Wun T, Law L, Harvey D, et al: Increased incidence of symptomatic venous thrombosis in patients with cervical carcinoma treated with concurrent chemotherapy, radiation, and erythropoietin. Cancer 98:1514-1520, 2003.
47. Vadhan-Raj S, Skibber JM, Crane C, et al: Randomized, double-blind, placebo-controlled trial of epoetin alfa (Procrit) in patients with rectal and gastric cancer undergoing chemo-radiotherapy (CT/RT) followed by surgery: early termination of the trial due to increased incidence of thromboembolic events (TEE) (abstract 2915). Blood 104:797a, 2004.
48. Kuter D: Thrombotic complications of central venous catheters in cancer patients. Oncologist 9:207-216, 2004.
49. Lopez JA, Kearon C, Lee AY: Deep venous thrombosis. Hematology (Am Soc Hematol Educ Program) 439-456, 2004.
50. Wermes C, von Depka Prondzinski M, Lichtinghagen R, et al: Clinical relevance of genetic risk factors for thrombosis in paediatric oncology patients with central venous catheters. Eur J Pediatr 158:S143-S146, 1999.
51. Fijnheer R, Paijmans B, Verdonck LF, et al: Factor V Leiden in central venous catherter-associated thrombosis. Br J Haematol 118:267-270, 2002.
52. Mandala M, Curigliano G, Bucciarelli P, et al: Factor V Leiden and G20210A prothrombin mutation and the risk of subclavian vein thrombosis in patients with breast cancer and a central venous catheter. Ann Oncol 15:590-593, 2004.
53. Mohren M, Markmann I, Dworschak U, et al: Thromboembolic complications after splenectomy for hematologic diseases. Am J Hematol 76:143-147, 2004.
54. Call GK, Bray PF, Smoker WR, et al: Carotid thrombosis following neck irradiation. Int J Radiat Oncol Biol Phys 18:635-640, 1990.
55. Carlson JW, Nazarian GK, Hartenbach E, et al: Management of pelvic venous stenosis with intravascular stainless steel stents. Gynecol Oncol 56:362-369, 1995.
56. Pihusch R, Danzl G, Scholz M, et al: Impact of thrombophilic gene mutations on thrombosis risk in patients with gastrointestinal carcinoma. Cancer 94:3120-3126, 2002.
57. Harrison CN, Donohoe S, Carr P, et al: Patients with essential thrombocythaemia have an increased prevalence of antiphospholipid antibodies which may be associated with thrombosis. Thromb Haemost 87:802-807, 2002.
58. Yoon KH, Wong A, Shakespeare T, et al: High prevalence of antiphospholipid antibodies in Asian cancer patients with thrombosis. Lupus 12:112-116, 2003.
59. Blom JW, Doggen CJM, Osanto S, et al: Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 293:715- 722, 2005.
60. Kennedy M, Andreescu ACM, Greenblatt MS, et al: Factor V Leiden, prothrombin 20210A and the risk of venous thrombosis among cancer patients. Br J Haematol 128:386-388, 2005.