The Timing of Chemotherapy-Induced Neutropenia and Its Clinical and Economic Impact
Chemotherapy-induced neutropenia (CIN) and its complications exact a substantial toll on patients with cancer. Febrile neutropenia (FN), a sign of life-threatening infections, is associated with lengthy hospitalizations, early mortality, and high medical costs. In addition, neutropenia is the primary cause of dose reductions and dose delays, limiting the delivery of the chemotherapy at full dose and on schedule and thus compromising long-term survival in patients with potentially curable malignancies. Many recent studies in several major tumor types have documented that the greatest risk of neutropenia and its complications is in the first cycle of chemotherapy, with more than 50% of the first episodes of neutropenia and FN occurring in the first cycle. In addition to their other negative effects, these first-cycle events are also associated with early termination of the chemotherapy. The disproportionately high risk of neutropenia in the first cycle has important implications for managing CIN, as well as for the development and use of guidelines for supportive care. It highlights the importance of determining which patients are at high risk for neutropenia and its complications before the chemotherapy is initiated and implementing interventions, such as prophylactic growth factor support in the first and subsequent cycles, to reduce that risk.
Chemotherapy-induced neutropenia (CIN) and its complications exact a substantial toll on patients with cancer. Febrile neutropenia (FN), a sign of life-threatening infections, is associated with lengthy hospitalizations, early mortality, and high medical costs. In addition, neutropenia is the primary cause of dose reductions and dose delays, limiting the delivery of the chemotherapy at full dose and on schedule and thus compromising long-term survival in patients with potentially curable malignancies. Many recent studies in several major tumor types have documented that the greatest risk of neutropenia and its complications is in the first cycle of chemotherapy, with more than 50% of the first episodes of neutropenia and FN occurring in the first cycle. In addition to their other negative effects, these first-cycle events are also associated with early termination of the chemotherapy. The disproportionately high risk of neutropenia in the first cycle has important implications for managing CIN, as well as for the development and use of guidelines for supportive care. It highlights the importance of determining which patients are at high risk for neutropenia and its complications before the chemotherapy is initiated and implementing interventions, such as prophylactic growth factor support in the first and subsequent cycles, to reduce that risk.
Chemotherapy-induced neutropenia (CIN) is the primary dose-limiting toxicity in patients with cancer treated with chemotherapy. It can lead to febrile neutropenia (FN), and it is associated with increased morbidity and early mortality, increased medical costs, and disruptions in potentially curative treatments.[1,2] In this article, I discuss the clinical and economic consequences of CIN and FN, as well as the risk and timing of CIN and FN in patients treated with myelosuppressive chemotherapy.
Consequences of Chemotherapy-Induced Neutropenia
Hospitalization, Mortality, and Costs
The incidences of CIN and its complications, such as fever, infection, and chemotherapy dose alterations, vary by type of malignancy. One large prospective registry reported CIN rates of 15% to 65% in patients with five major tumor types: breast cancer, colon cancer, lymphoma, lung cancer, and ovarian cancer. The rates of FN ranged from 7% to 30%.[3]
Hospitalization and treatment with empiric broad-spectrum antibiotics is typically required in patients with FN, and it has been estimated that in the United States more than 60,000 patients a year are hospitalized for FN.[4]
These hospitalizations account for substantial mortality and medical costs. A review of discharge data from the University HealthSystem Consortium (UHC), which comprises 121 institutions, examined inpatient mortality in 41,779 nontransplant patients who had been hospitalized for FN in 1995 through 2000 and found that it was higher in patients with leukemia than in those with lymphoma and solid tumors (Figure 1).[1] The length of hospitalization followed a similar pattern, averaging 19.0 days in patients with leukemia, 10.8 days in those with lymphoma, and 8.5 days in those with solid tumors.[1] The average cost of hospitalization was $37,600 in patients with leukemia, $19,000 in patients with lymphoma, and $12,300 in patients with solid tumors.[1]
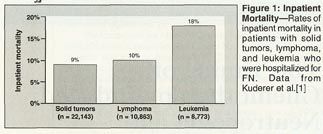
A minority of patients in the UHC database accounted for a disproportionate amount of medical costs. The mean cost of hospitalization in patients with complications that were related to FN was nearly double the mean cost in all patients ($18,400 vs $35,000). For some patients, the hospital stays were 30 to 40 days, costing as much as $60,000 to $100,000 for a single hospitalization for FN.[1]
It should be noted that the medical costs in the UHC discharge records are only the direct costs of hospitalization and that other costs incurred by patients can substantially increase the total costs of an occurrence of FN. A survey of 26 patients with ovarian cancer in whom World Health Organization grade 3 or 4 CIN had occurred captured both its direct and its indirect costs in the 3 months after its occurrence.[5] The direct costs included out-of-pocket expenses such as physician visits, emergency room visits, drugs and medical devices, home healthcare, and laboratory tests. The indirect costs included patient work loss, family member work loss, and caregiver payments. The total direct costs were estimated at $1,340 and the total indirect costs at $3,840.
Patient Quality of Life
There is increasing evidence that neutropenia is also associated with important-although more difficult to measure-effects on patient quality of life (QOL). Hospitalization for FN and the fear of hospitalization have obvious negative effects on patient QOL,[6] but the effects of neutropenia are more subtle. Fortner and colleagues used several tools to evaluate the effects of CIN and FN on QOL in a heterogeneous group of patients. Lower absolute neutrophil counts were found to correlate with lower ability for physical work, worse physical symptoms, and greater psychological distress.[7,8] In another study, which analyzed 100 structured interviews with 34 patients in whom grade 4 neutropenia developed in the first cycle of chemotherapy, fatigue was the most common physical symptom reported, and interference with daily routine, negative self-evaluation, negative emotion, and social isolation were other common complaints that were associated with neutropenia.[9] A possible association between FN and other adverse events has also been shown, with the incidence and severity of common side effects such as nausea and vomiting, anorexia, and asthenia being increased in patients with FN.[10-12] When prophylactic growth factors were used to reduce the incidence of FN, the incidence of other adverse events was also reduced.[12]
Reduced Dose Intensity
The most insidious effect of CIN may, however, be the disruption of the cancer treatment-with potentially serious consequences for long-term survival. Dose reductions and delays are common in patients treated with chemotherapy. A retrospective analysis of data on 20,799 patients who were treated with chemotherapy after surgery for early-stage breast cancer between 1997 and 2000 found that dose delays of 7 days or longer were required in 25% of patients, dose reductions of 15% or more were required in 37%, and relative dose intensity (RDI) was less than 85% in 56% (Figure 2).[13] A similar analysis of data on 4,522 patients who were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), CHOP plus rituximab (Rituxan) (CHOP-R), or CNOP (mitoxantrone [Novantrone] substituted for doxorubicin) for aggressive non-Hodgkin's lymphoma (NHL) found dose delays of 7 days or longer in 24% of patients, dose reductions of 15% or more in 40%, and RDI less than 85% in 53%. [14] Multivariate analyses in both studies found that advanced age (≥ 60 or ≥ 65 years) was a significant predictor of RDI less than 85%, independent of other risk factors such as poor Eastern Cooperative Oncology Group performance status, advanced tumor stage, increased body surface area, and absence of treatment with CSFs.[13,14]
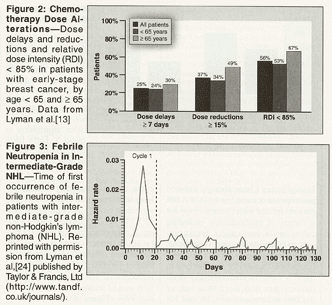
Most clinicians would name CIN and its complications as the most frequent causes of chemotherapy dose delays and dose reductions, and recent data support this. A retrospective analysis of practice patterns examined regimen alterations in 1,111 patients who were treated with adjuvant therapy with cyclophosphamide, methotrexate, and fluorouracil (CMF), doxorubicin and cyclophosphamide (AC), or other regimens for early-stage breast cancer. Across all regimens, the RDI was less than 85% of that planned in 22% of patients. Chemotherapy-induced neutropenia was reported as the most frequent cause of the dose reductions and delays, accounting for 61% of these regimen alterations.[15] An analysis of similar data from 492 patients treated with CHOP or CNOP for intermediate-grade NHL had similar findings. With both regimens combined, there were dose reductions or delays in 48% of patients; CIN was once again the most frequent cause, accounting for 56% of the delays and 59% of the reductions.[16] There were similar findings in a recent trial of vinorelbine plus cisplatin in 242 patients with resected non-small-cell lung cancer. World Health Organization grade 3 or 4 neutropenia occurred in 73% of patients and FN occurred in 7%. There was at least one dose delay in 55% of patients, generally due to persistent neutropenia at the time of the planned dose of vinorelbine on day 15 of the cycle.[17]
Such alterations in chemotherapy regimens may help ameliorate the immediate problem of neutropenia, but their long-term effects are clearly negative. The most recent update of the classic study by Bonadonna and colleagues well illustrates this point. This study, a retrospective analysis of data on 207 patients treated with 12 cycles of CMF for node-positive breast cancer, found significantly lower long-term survival in patients who were treated with less than 85% of the planned dose. At a median follow-up of 28.5 years, relapse-free survival was 26% in patients treated with less than 85% of the planned dose and 42% in those treated with a higher RDI, and overall survival was 21% and 40%.[18] Analysis of data on another frequently curable malignancy, previously untreated NHL, had similar results. In 115 patients treated with CHOP, methotrexate, bleomycin (Blenoxane), doxorubicin, cyclophosphamide, vincristine, and dexamethasone ([M]BACOD), or methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin (MACOP-B) chemotherapy, a doxorubicin RDI of 75% or less in the first three cycles was found to be the strongest predictor of mortality at a median follow-up of 3 years (P = .001). Even when the analysis was restricted to patients in whom a complete response had been achieved, a low RDI of doxorubicin remained a predictor of poor survival.[19] In addition to these frequently curable tumors, alterations in chemotherapy regimens appear to also compromise the treatment outcomes in settings in which treatment is less commonly curative. In a randomized trial of full-dose and attenuated-dose etoposide and cisplatin in 95 patients with small-cell lung cancer, the response rate was lower in patients treated with the attenuated dose (39% vs 68%) as was 1-year survival (18% vs 39%).[20]
Perhaps the best evidence for the importance of delivered chemotherapy dose intensity comes from Cancer and Leukemia Group B (CALGB) 9741, a large clinical trial in women with node-positive early-stage breast cancer that tested the regimen of concurrent doxorubicin and cyclophosphamide followed by paclitaxel (AC-T) given in dose-dense 14-day cycles and in standard 21-day cycles.[21] To reduce the risk of neutropenia, proactive CSFs were given to all patients treated with the 14-day regimen. The protocol-specified 36-month analysis showed significantly higher disease-free survival (risk ratio 0.74, P = .010) and overall survival (risk ratio 0.69, P = .013) in the patients treated with the 14-day regimen. Five-year results were recently reported, with these differences in survival remaining significant and of similar magnitude.[22] On the basis of these results, many specialists in breast cancer have adopted dose-dense AC-T with routine growth factor support as a standard regimen in women with node-positive early-stage breast cancer. Many are also using once-per-cycle pegfilgrastim rather than daily filgrastim, a strategy that has been shown to be safe and effective.[23]
Risk in the First Cycle
A growing body of evidence supports the conclusion that, contrary to common belief, the risk of CIN and FN is highest in the first cycle of chemotherapy rather than cumulative across cycles. An analysis of data on 577 patients with intermediate-grade NHL treated with up to six cycles of CHOP found that more than half of the first occurrences of FN-58% were in the first cycle (Figure 3).[24] Similar results were seen in a randomized trial of CHOP and CHOP-R in 528 patients with NHL, in which the largest proportion of all occurrences of FN, 39%, were in the first cycle of the chemotherapy.[25] In both studies, most of the occurrences of FN in the first cycle were by day 14.
A similar pattern has been seen in a large prospective registry, the Awareness of Neutropenia in Chemotherapy Registry, which collects data on patients treated with chemotherapy at 137 clinical sites across the United States. Chemotherapy-induced neutropenia occurred in 43% of 2,106 patients treated with chemotherapy for a variety of malignancies, including colon cancer, lung cancer, breast cancer, ovarian cancer, and lymphoma, and FN occurred in 14%. The likelihoods of CIN and FN were highest in the first cycle of chemotherapy in all tumor types, and more than half of the first occurrences of CIN and FN were in the first cycle.[3]
There are several possible reasons for the risk of CIN and FN being highest in the first cycle, and the relative lack of supportive care in the first cycle is an important one. In patients with NHL treated with CHOP or CHOP-R, for example, 41% of patients who were not given CSFs in the first and subsequent cycles experienced FN.[25] In addition, a number of studies have shown that the absence of proactive CSF therapy is a significant predictor of FN.[16,24,26] Whatever the cause of risk in the first cycle, CIN that occurs in the first cycle has the potential for severe consequences. In a study in 267 elderly patients with NHL treated with CHOP, there were 35 treatment-related deaths. The majority of these deaths, 63%, occurred in the first cycle, and 82% of all treatment-related deaths were infection-related.[27]
The occurrence of CIN in the first cycle also substantially affects the long-term course of treatment. There were dose reductions or delays in 61% of 1,111 patients treated with a variety of chemotherapy regimens for early-stage breast cancer, and the occurrence of one such treatment alteration predicted later alterations, the incidence of additional delays being 57% and of additional reductions being 79% (Figure 4).[15] The same pattern was seen in 492 patients with intermediate-grade NHL who were treated with CHOP or CNOP, the incidence of additional delays being 41% and of additional reductions being 68% (see Figure 4).[16] What is also striking in the Picozzi study is the apparent underuse of CSFs to prevent later dose reductions and delays, even though the majority of those treatment alterations were necessitated by the occurrence of neutropenia. Only 2% to 3% of patients in whom there was a dose delay or reduction were subsequently treated with CSFs to lessen the likelihood of later delays or reductions.[16]
Chemotherapy-induced neutropenia in the first cycle is also associated with premature termination of the chemotherapy. In a study in 124 patients with intermediate-grade NHL, treatment with six to eight cycles of CHOP was planned-an optimal number of cycles-but 20% of the patients were treated with fewer than six cycles, and those patients who were hospitalized for FN in the first cycle were more than four times as likely to be treated with fewer than six cycles as were those who were not hospitalized for FN.[28]
That the occurrence of neutropenia in the first cycle can compromise the course of cancer treatment should be considered in the context of treatment efficacy and long-term survival. In the study of 124 patients with NHL discussed above, overall survival at 5 years was significantly lower in patients aged 60 to 74 years who were treated with fewer than six cycles of CHOP (hazard ratio for death, 6.0; 95% CI, 2.4-15.2). Overall survival at 5 years was also lower in the patients aged 75 years and older who were treated with fewer than six cycles, but the number of patients was too small for significance to be shown.[28] As discussed earlier, treatment with less-than-optimal RDI has also been associated with significantly lower long-term survival in much larger studies.[18,19,21]
Conclusions
Chemotherapy-induced neutropenia is a major dose-limiting toxicity in patients treated with chemotherapy, with significant clinical consequences in terms of increased morbidity and early mortality.[1,2] Hospitalization is often required in patients with FN, and it can be both lengthy and expensive.[1] In some high-risk patients in whom serious infections occur, mortality can exceed 50% even with prompt anti-infective treatment.[29,30] The most effective approach to managing neutropenia, therefore, is to prevent FN.
As discussed by Rader in this supplement,[31] effective supportive care measures such as CSFs are available for managing neutropenia. Treatment with CSFs can not only reduce the incidence of FN but also reduce infections and increase the proportion of patients who are treated with chemotherapy at full dose and on schedule.[26]
The finding that FN occurs most often in the first cycle of chemotherapy has important implications for the use of supportive care in oncology, and in particular for the use of therapies for managing neutropenia and the continuing development of clinical guidelines for their use. As discussed by Crawford in this supplement,[32] patients who are at increased risk for FN should be treated with CSFs starting in the first cycle of chemotherapy.
Disclosures:
Dr. Ozer has served on speakers bureaus for and received research grants from Amgen, Genentech, and Sanofi-Aventis, and he also has received grants from Lilly and Novartis.
References:
1. Kuderer NM, Dale DC, Crawford J, et al: Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer In press.
2. Lyman GH: Guidelines of the National Comprehensive Cancer Network on the use of myeloid growth factors with cancer chemotherapy: A review of the evidence. J Natl Comp Cancer Netw 3:557-571, 2005.
3. Crawford J, Wolff DA, Culakova E, et al: First cycle risk of severe and febrile neutropenia in cancer patients receiving systemic chemotherapy: Results from a prospective nationwide study (abstract 2210). Blood 104:607a-608a, 2004.
4. Caggiano V, Weiss RV, Rickert TS, et al: Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916-1924, 2003.
5. Cosler LE, Calhoun EA, Agboola O, et al: Effects of indirect and additional direct costs on the risk threshold for prophylaxis with colony-stimulating factors in patients at risk for severe neutropenia from cancer chemotherapy. Pharmacotherapy 24:488-494, 2004.
6. Lyman GH, Kuderer NM: Filgrastim in patients with neutropenia. Potential effects on quality of life. Drugs 62(suppl 1):65-78, 2002.
7. Fortner BV, Stolshek B, Schwartzberg LS, et al: Decline in absolute neutrophil count (ANC) is associated with lower quality of life (QOL) in cancer patients receiving docetaxel (abstract 2808). Proc Am Soc Clin Oncol 21:247b, 2002.
8. Okon TA, Fortner BV, Schwartzberg L, et al: Quality of life (QOL) in patients with grade IV chemotherapy-induced neutropenia (CIN) (abstract 2920). Proc Am Soc Clin Oncol 21:275b, 2002.
9. Fortner BV, Tauer KW, Okon T, et al: Experiencing neutropenia: Quality of life interviews with adult cancer patients. BMC Nurs 4:4, 2005.
10. Glaspy J, Hackett J, Flyer P, et al: Febrile neutropenia is associated with an increase in the incidence, duration, and severity of chemotherapy toxicities (abstract 1812). Blood 98:432a, 2001.
11. Martin M, Lluch A, Segui MA, et al: Prophylactic growth factor (GF) support with adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) for node-negative breast cancer (BC): An interim safety analysis of the GEICAM 9805 study (abstract 620). Proc Am Soc Clin Oncol 23:32, 2004.
12. Martin M, Lluch A, Seguà MA, et al: Toxicity and health-related quality of life (HRQoL) in node-negative breast cancer (BC) patients (pts) receiving adjuvant treatment with TAC (docetaxel, doxorubicin, cyclophoshamide) or FAC (5-fluorouracil, doxorubicin, cyclophosphamide): Impact of adding prophylactic growth factors (GF) to TAC. GEICAM study 9805 (abstract 604) J Clin Oncol 23(suppl):29s, 2005.
13. Lyman GH, Dale DC, Crawford J: Incidence and predictors of low dose intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol 21:4524-4531, 2003.
14. Lyman GH, Dale DC, Friedberg J, et al: Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: A nationwide study. J Clin Oncol 22:4302-4311, 2004.
15. Link BK, Budd GT, Scott S, et al: Delivering adjuvant chemotherapy to women with early-stage breast carcinoma: Current patterns of care. Cancer 92:1354-1367, 2001.
16. Picozzi VJ, Pohlman BL, Morrison VA, et al: Patterns of chemotherapy administration in patients with intermediate-grade non-Hodgkin's lymphoma. Oncology (Williston Park) 15:1296-1306, 2001.
17. Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med 352:2589-2597, 2005.
18. Bonadonna G, Moliterni A, Zambetti M, et al: 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: Cohort study. BMJ 330:217, 2005.
19. Kwak LW, Halpern J, Olshen RA, et al: Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: Results of a tree-structured survival analysis. J Clin Oncol 8:963-977, 1990.
20. Ardizzoni A, Favaretto A, Boni L, et al: Platinum-etoposide chemotherapy in elderly patients with small-cell lung cancer: Results of a randomized multicenter phase II study assessing attenuated-dose or full-dose with lenograstim prophylaxis-a Forza Operativa Nazionale Italiana Carcinoma Polmonare and Gruppo Studio Tumori Polmonari Veneto (FONICAP-GSTPV) study. J Clin Oncol 23:569-575, 2005.
21. Citron ML, Berry DA, Cirrincione C, et al: Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup trial C9741/Cancer and Leukemia Group B trial 9741 [erratum in J Clin Oncol 21:2226, 2003]. J Clin Oncol 21:1431-1439, 2003.
22. Hudis C, Citron M, Berry D, et al: Five year follow-up of INT C9741: Dose-dense (DD) chemotherapy (CRx) is safe and effective (abstract 41). Presented at the 28th Annual San Antonio Breast Cancer Symposium, December 8-11, 2005, San Antonio, Texas.
23. Burstein HJ, Parker LM, Keshaviah A, et al: Efficacy of pegfilgrastim and darbepoetin alfa as hematopoietic support for dose-dense every-2-week adjuvant breast cancer chemotherapy. J Clin Oncol 23:8340-8347, 2005.
24. Lyman GH, Morrison VA, Dale DC, et al: Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44:2069-2076, 2003.
25. Morrison VA, Weller EA, Habermann TM, et al: Patterns of growth factor (GF) usage and febrile neutropenia (FN) among older patients (pts) with diffuse large B-cell lymphoma (DLBCL) treated with CHOP or R-CHOP: An Intergroup Experience (CALGB 9793, ECOG-SWOG 4494) (abstract 3309). Blood 104:904a, 2004.
26. Kuderer NM, Crawford J, Dale DC, et al: Meta-analysis of prophylactic granulocyte colony-stimulating factor (G-CSF) in cancer patients receiving chemotherapy (abstract 8117). J Clin Oncol 23(suppl):758s, 2005.
27. Gomez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin's lymphoma: Results of a multivariate analysis. J Clin Oncol 16:2065-2069, 1998.
28. Chrischilles EA, Link BK, Scott SD, et al: Factors associated with early termination of CHOP therapy and the impact on survival among patients with chemosensitive intermediate-grade non-Hodgkin's lymphoma. Cancer Control 10:396-403, 2003.
29. Lyman GH, Kuderer NM: Epidemiology of febrile neutropenia. Support Cancer Ther 1:1-12, 2003.
30. Lyman GH, Kuderer NM: Cost effectiveness of myeloid growth factors in cancer chemotherapy. Curr Hematol Rep 2:471-479, 2003.
31. Rader M: Granulocyte colony-stimulating factors use in patients with chemotherapy-induced neutropenia: clinical and economic benefits. Oncology (suppl) TK:TK-TK, 2006. (this supplement)
32. Crawford J: Risk assessment and guidelines for first-cycle colony-stimulating factor use in the management of chemotherapy-induced neutropenia. Oncology (suppl) TK:TK-TK, 2006. (this supplement)