Topotecan in Combination With Cisplatin for the Treatment of Stage IVB, Recurrent, or Persistent Cervical Cancer
Topotecan, a camptothecin analog previously approved for the treatment of ovarian cancer and small-cell lung cancer, was granted regular approval by the US Food and Drug Administration (FDA) on June 14, 2006, for use in combination with cisplatin to treat women with stage IVB, recurrent, or persistent carcinoma of the cervix not amenable to curative treatment with surgery and/or radiation therapy. The purpose of this summary is to review the database supporting this approval.
Purpose: Topotecan, a camptothecin analog previously approved for the treatment of ovarian cancer and small-cell lung cancer, was granted regular approval by the US Food and Drug Administration (FDA) on June 14, 2006, for use in combination with cisplatin to treat women with stage IVB, recurrent, or persistent carcinoma of the cervix not amenable to curative treatment with surgery and/or radiation therapy. The purpose of this summary is to review the database supporting this approval.
Experimental Design: In a randomized multicenter study enrolling 293 eligible patients, topotecan plus cisplatin (TC) was compared with cisplatin monotherapy. The TC regimen consisted of cisplatin 50 mg/m2 IV over 1 hour on day 1 and topotecan 0.75 mg/m2 IV over 30 minutes on days 1, 2, and 3 every 21 days.
Results: There was a clinically relevant and statistically significant improvement in overall survival in the TC treatment arm. Median overall survival was 9.4 months (95% confidence interval [CI]:7.9-11.9) in the TC arm, compared to 6.5 months (95% CI:5.8-8.8) with cisplatin alone. The unadjusted hazard ratio for overall survival between treatment arms was 0.76 (95% CI: 0.59-0.98, P = .033) favoring the combination arm. The most common toxicities with TC included myelosuppression, nausea and vomiting, mucositis, rash, and hepatotoxicity.
Conclusions: This report describes the FDA's review supporting this first approval of a chemotherapeutic drug for advanced cervical cancer based on demonstration of a survival benefit.
Topotecan (Hycamtin) is an antineoplastic agent that acts by inhibiting the activity of topoisomerase I, a cellular enzyme that relieves torsional strain in DNA by inducing reversible single-strand breaks. Topotecan binds to the enzyme-DNA complex and prevents religation of these single-strand breaks. Its cytotoxicity is thought to be due to the accumulation of double-strand DNA damage, which mammalian cells cannot efficiently repair.
Topotecan has been previously approved by the US Food and Drug Administration (FDA) for patients with metastatic carcinoma of the ovary after failure of initial or subsequent chemotherapy and for the treatment of sensitive small-cell lung cancer after failure of first-line chemotherapy.
Stage IVB, recurrent, or persistent carcinoma of the cervix not amenable to curative treatment with surgery and/or radiation therapy is associated with an estimated median survival of 7 to 10 months.[1-3] No single agent or combination regimen has previously shown a survival benefit in this setting, and no chemotherapeutic drug or drug combinations have been approved by the FDA for this patient population.
Cisplatin appears to be the most active single-agent chemotherapy regimen for this indication, based on response rates of 21% to 31%.[4] Combination chemotherapy regimens generally produce higher response rates and longer progression-free survival (PFS) than cisplatin monotherapy in this population. However, toxicity is greater with no statistically significant improvement in overall survival.[5-16]
Topotecan has been evaluated using a daily × 5 schedule, either every 3 weeks or every 4 weeks, in single-arm studies for patients with cervical cancer that was refractory to or had relapsed following cisplatin-based chemotherapy. Objective overall response rates of 12% to 19% have been noted.[17-19]
GOG-0179 was a Gynecologic Oncology Group (GOG)-sponsored study conducted at 94 study centers in the United States. It was designed to compare topotecan plus cisplatin (TC) with a control arm of cisplatin monotherapy. The design and findings of this study are outlined below.
Experimental Design
Study Population
The main protocol-specified inclusion criteria included histologically or cytologically confirmed International Federation of Gynecology and Obstetrics stage IVB, recurrent, or persistent squamous cell, adenosquamous, or adenocarcinoma of the cervix not amenable to curative treatment with surgery and/or radiotherapy, measurable disease by physical examination or imaging, GOG performance status 0-2, and adequate bone marrow, renal, and hepatic function. Except for use as radiosensitization, prior chemotherapy was not allowed. Patients with bilateral hydronephrosis not amenable to decompression or with known brain or leptomeningeal involvement were excluded.
Objectives and Statistical Plan
The primary efficacy endpoint was overall survival, defined as time from randomization to the date of death from any cause. The final efficacy analysis was planned to be conducted after a total of 111 deaths were observed in the cisplatin monotherapy group. The study cutoff date for the primary analysis was October 31, 2003, the date on which the sponsor received notification of the 129th event. All patients known to be alive at the cutoff date were censored either on the date of last assessment or on the cutoff date if the last contact had taken place subsequently. In order to account for one early interim analysis for futility, the nominal two-sided significance level for the final analysis was set at 0.044. The unstratified log-rank test was prospectively selected as the primary means of determining whether TC increased overall survival compared with cisplatin monotherapy.
Treatment Plan
Patients were randomized in a 1:1 ratio to receive either TC or cisplatin monotherapy. Patients randomized to TC received topotecan 0.75 mg/m2 intravenously over 30 minutes on days 1, 2, and 3, after hydration and premedication with granisetron (Kytril) and dexamethasone, followed by cisplatin 50 mg/m2 in 500 mL 3% saline intravenously over 1 hour on day 1. Patients randomized to cisplatin received 50 mg/m2 in 500 mL 3% saline intravenously over 1 hour on day 1.
A third treatment arm consisting of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) was closed after 64 patients had been enrolled due to excess toxic deaths. Data from patients enrolled to that arm were not submitted with this application.
Patients in each arm were to continue treatment for six cycles or until disease progression or unacceptable toxicity. Patients in either group with objective responses and acceptable toxicity were permitted to continue their assigned treatment beyond six cycles after discussion with the study chair.
Patient Population/ Demographics
A total of 300 patients (150 per arm) were randomized to receive TC vs cisplatin monotherapy. The primary efficacy analysis was conducted on a modified intent-to-treat population (n = 293), consisting of all of the randomized patients with the exception of seven who were subsequently found not to meet major criteria for enrollment. The safety database consisted of 284 patients who received at least one dose of study drug (140 of whom received TC).
Median patient ages were 46 and 48 years in the TC and cisplatin arms, respectively. In each treatment arm, over 70% of patients were white and almost half were GOG performance status 0.
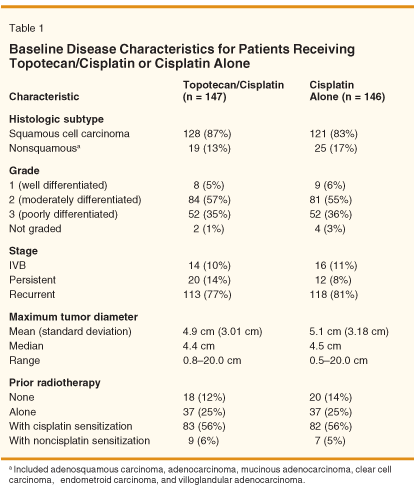
Baseline disease characteristics and prior therapy of the study population are listed in Table 1 and are evenly distributed across treatment groups.
Efficacy
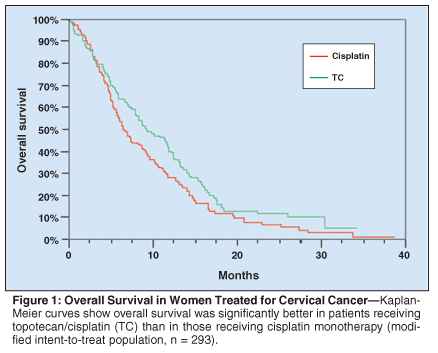
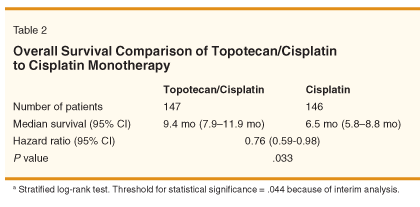
Overall survival was significantly superior in the TC group compared with the cisplatin monotherapy group (see Table 2 for summary of results and Figure 1 for Kaplan-Meier curves).
The Applicant (GlaxoSmithKline) claimed TC was also superior to cisplatin for progression-free survival (PFS) and overall response rate. The FDA considered these secondary endpoints as exploratory and not supportive of a marketing claim because the Applicant did not submit tumor measurements or the means by which individual relapses were detected (eg, by symptoms, physical findings, or radiographically). Therefore, the FDA could not verify the claimed responses or PFS findings. In addition, there was no prespecified plan for adjustment for multiplicity or ordering of secondary analyses of these endpoints.
Safety
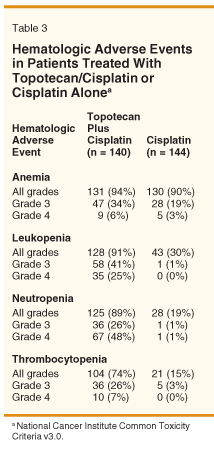
Myelosuppression is the chief dose-limiting toxicity with TC. Patient-incidences in GOG-0179 of grade 3/4 neutropenia, anemia, and thrombocytopenia were higher in the TC treatment arm compared to the cisplatin-alone arm (see Table 3 for details). Grade 3/4 neutropenia occurred in 19% of patients receiving TC compared to 8% for patients receiving cisplatin alone, and grade 3/4 hemorrhage occurred in approximately 7% of patients receiving TC compared to 3% receiving cisplatin alone. Grade 3/4 hematologic toxicity did not appear to be cumulative because most patients continued to receive topotecan at protocol-directed reduced doses (data not shown).
Toxicity categories for which grade 3/4 nonhematologic adverse events were reported more commonly in the TC treatment arm than with cisplatin monotherapy were gastrointestinal (nausea, vomiting, stomatitis-pharyngitis, and other; 45% vs 28%), pain (22% vs 16%), metabolic-laboratory (14% vs 10%), and hepatic (5% vs 1%). Dermatologic toxicity was reported more frequently in the TC treatment arm (48% vs 20%), but fewer than 1% of episodes were grade 3 or 4.
The incidence of deaths within 30 days of last treatment infusion was equally distributed across treatments: 10% for TC, and 8% for cisplatin monotherapy. Most were attributed to malignant disease or "other" causes. Of the deaths occurring within 30 days of last infusion, four in the TC arm and two on cisplatin monotherapy were attributed to drug toxicity.
Drug-Drug Interactions
Two studies examined the potential for drug-drug interactions between topotecan and cisplatin. The results of these studies showed that topotecan does not affect the pharmacokinetics of cisplatin and vice versa.
Special Populations
Approximately 7% of the GOG-0179 study population was 65 years of age or older and 28% were nonwhite. The limited data generated by these segments of the study population suggest that initial topotecan doses need not be adjusted for age or race and that efficacy in older and nonwhite patients is comparable to the overall population. Trends toward improved survival were seen for the TC treatment arm in both age (≥ 65 vs < 65 years) and race (white vs nonwhite) subgroups.
GOG-0179 excluded patients with renal insufficiency (baseline serum creatinine >1.5 mg/dL) and hepatic insufficiency (serum bilirubin > 1.5 * institutional normal and aspartate aminotransferase and alkaline phosphatase > 3 * institutional normal). This application therefore provided no data on safety and efficacy for patients with impaired renal or hepatic function.
Discussion
Although several single-agent and combination regimens have previously shown antitumor activity in stage IVB, recurrent, or persistent cervical cancer, none have shown a survival benefit in this setting. The results of GOG-0179 show a survival benefit of topotecan given every 3 weeks with cisplatin in this setting. The findings reported herein provide a rationale for investigating the use of topotecan in cervical cancer patients with less advanced disease. These results also warrant additional investigation of novel agents in patients with cervical cancer who have advanced disease and who progress after surgery and/or radiotherapy.
The topotecan dose and schedule recommended in the product label for single-agent therapy of metastatic ovarian carcinoma or small-cell lung cancer is 1.5 mg/m2 daily for 5 days every 21 days. Of three phase II studies evaluating single-agent topotecan for patients with cervical cancer that was refractory to or had relapsed following cisplatin-based chemotherapy, one used the dose and schedule recommended by the product label as single-agent therapy for metastatic ovarian carcinoma or small-cell lung cancer.[17] The other two reduced the dose intensity by altering the dose size and cycle interval.[18-19] The reason for this adjustment was that women with relapsed/refractory cervical cancer often have compromised bone marrow reserve because of prior pelvic irradiation.
The maximum tolerated dose in the initial phase I studies of topotecan with cisplatin on a daily × 5 every 21 days schedule was established as topotecan 0.75 mg/m2 daily × 5 and cisplatin 50 mg/m2 on day 1. Neutropenia and thrombocytopenia were dose-limiting.[20]
Administration of cisplatin before topotecan was significantly more myelosuppressive than the alternate sequence, regardless of filgrastim (Neupogen) support. In vitro data failed to explain the differential toxicity of these two sequences. Rather, pharmacokinetic studies suggested that its basis was reduced topotecan clearance when preceded by cisplatin, possibly due to subclinical renal tubular toxicity of cisplatin. The sequence of cisplatin before topotecan at doses of 50 and 0.75 mg/m2, respectively, was recommended for phase II development.[21]
The GOG-0179 design anticipated the effects of both prior pelvic irradiation and sequence dependence. The schedule specified that topotecan be administered before cisplatin and that the number of topotecan doses per cycle be reduced from five to three.
Previous pharmacokinetic studies suggest that doses of intravenous topotecan should not need adjustment based on gender.[22,23] Prior clinical studies have not included sufficient numbers of patients age 65 and over to determine whether they respond differently than younger patients. The current product label advises full initial doses for patients of either gender or with hepatic insufficiency and initial dose adjustment for patients with moderate renal insufficiency (creatinine clearance: 20-40 mL/min). The product label advises cautious dose selection for elderly patients, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy in that population.
The safety and efficacy of topotecan in persons under age 18 have not been established, as the Agency granted the Applicant waivers for pediatric studies in earlier topotecan applications. Cervical cancer is almost nonexistent in children,[24] and GOG-0179 enrolled no pediatric patients. The Applicant requested a waiver for pediatric studies with topotecan with the current efficacy supplement, and the Agency granted this waiver.
Conclusions
On June 14, 2006, the FDA approved topotecan for use in combination with cisplatin for the treatment of women with stage IVB, recurrent or persistent carcinoma of the cervix not amenable to curative treatment with surgery and/or radiation therapy. This approval was based on a survival advantage shown over cisplatin monotherapy in a multicenter GOG-sponsored clinical trial. The recommended dose of topotecan 0.75 mg/m2 by intravenous infusion over 30 minutes on days 1, 2, and 3; followed by cisplatin 50 mg/m2 by intravenous infusion on day 1 repeated every 21 days according to bone marrow recovery.
The most common toxicities observed with TC were myelosuppression, nausea and vomiting, mucositis, rash, and hepatotoxicity. These toxicities had previously been described with topotecan. Although myelosuppression was greater in the TC arm, bone marrow toxicity did not appear to be cumulative and was generally manageable. No new categories of toxicity were identified with the addition of cisplatin.
Disclosures:
>br />Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Vermorken JB, Zanetta G, De Oliveira CF, et al: Randomized phase III trial of bleomycin, vindesine, mitomycin-C, and cisplatin (BEMP) versus cisplatin (P) in disseminated squamous-cell carcinoma of the uterine cervix: an EORTC Gynecological Cancer Cooperative Group study. Ann Oncol 12:967-974, 2001.
2. Moore DH, Blessing JA, McQuellon RP, et al: Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol 22:3113-3119, 2004.
3. Omura GA, Blessing JA, Vaccarello L, et al: Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol 15:165-171, 1997.
4. Bonomi P, Blessing JA, Stehman FB, et al: Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: A Gynecologic Oncology Group Study. J Clin Oncol 3:1079-1085, 1985.
5. Bloss JD, Blessing JA, Behrens BC, et al: Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol 20:1832-1837, 2002.
6. Rose PG, Blessing JA, Gershenson DM, et al: Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol 17:2676-2680, 1999.
7. Dimopoulos MA, Papadimitriou CA, Sarris K, et al: Combination of ifosfamide, paclitaxel, and cisplatin for the treatment of metastatic and recurrent carcinoma of the uterine cervix: A phase II study of the Hellenic Cooperative Oncology Group. Gynecol Oncol 85:476-482, 2002.
8. Burnett AF, Roman LD, Garcia AA, et al: A phase II study of gemcitabine and cisplatin in patients with advanced, persistent, or recurrent squamous cell carcinoma of the cervix. Gynecol Oncol 76:63-66, 2000.
9. Wagenaar HC, Pecorelli S, Mangioni C, et al: Phase II study of mitomycin-C and cisplatin in disseminated, squamous cell carcinoma of the uterine cervix. A European Organization for Research and Treatment of Cancer (EORTC) Gynecological Cancer Group study. Eur J Cancer 37:1624-1628, 2001.
10. Morris M, Blessing JA, Monk BJ, et al: Phase II study of cisplatin and vinorelbine in squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol 22:3340-3344, 2004.
11. Muggia FM, Blessing JA, McGehee R, et al: Cisplatin and irinotecan in squamous cell carcinoma of the cervix: A phase II study of the Gynecologic Oncology Group. Gynecol Oncol 94:483-487, 2004.
12. Pohlmann P, DiLeone LP, Cancella AI, et al: Phase II trial of cisplatin plus decitabine, a new DNA hypomethylating agent, in patients with advanced squamous cell carcinoma of the cervix. Am J Clin Oncol 25:496-501, 2002.
13. Tinker AV, Bhagat K, Swenerton KD, et al: Carboplatin and paclitaxel for advanced and recurrent cervical carcinoma: The British Columbia Cancer Agency experience. Gynecol Oncol 98:54-58, 2005.
14. Sit AS, Kelley JL, Gallion HH, et al: Paclitaxel and carboplatin for recurrent or persistent cancer of the cervix. Cancer Invest 22:368-373, 2004.
15. Nagao S, Fujiwara K, Oda T, et al: Combination chemotherapy of docetaxel and carboplatin in advanced or recurrent cervix cancer. A pilot study. Gynecol Oncol 96:805-809, 2005.
16. Umesaki N, Fujii T, Nishimura R, et al: Phase II study of irinotecan combined with mitomycin-C for advanced or recurrent squamous cell carcinoma of the uterine cervix: The JGOG study. Gynecol Oncol 95:127-132, 2004.
17. Bookman MA, Blessing JA, Hanjani P, et al: Topotecan in squamous cell carcinoma of the cervix: A phase II study of the Gynecologic Oncology Group. Gynecol Oncol 77:446-449, 2000.
18. Muderspach LI, Blessing JA, Levenback C, et al: A phase II study of topotecan in patients with squamous cell carcinoma of the cervix: A Gynecologic Oncology Group Study. Gynecol Oncol 81:213-215, 2001.
19. Abu-Rustum NR, Lee S, Massad LS: Topotecan for recurrent cervical cancer after platinum-based therapy. Int J Gynecol Cancer 10:285-288, 2000.
20. Raymond E, Burris HA, Rowinsky EK, et al: Phase I study of daily times five topotecan and single injection of cisplatin in patients with previously untreated non-small-cell lung carcinoma. Ann Oncol 8:1003-1008, 1997.
21. Rowinsky EK, Kaufmann SH, Baker SD, et al: Sequences of topotecan and cisplatin: phase I, pharmacologic, and in vitro studies to examine sequence dependence. J Clin Oncol 14:3074-3084, 1996.
22. Loos WJ, Gelderblom HJ, Verweij J, et al: Gender dependent pharmacokinetic of topotecan in adult patients. Anticancer Drugs 11:673-680, 2000.
23. Mould DR, Holford NH, Schellens, et al: Population pharmacokinetic and adverse event analysis of topotecan in patients with solid tumors. Clin Pharmacol Ther 71:334-348, 2002.
24. Available at http://seer.cancer.gov/statfacts/html/cervix.html. Accessed Sept 9, 2006.