Trastuzumab-DM1 delivers encouraging results in HER2+ breast cancer
Impressive results from an ongoing study of an anti-HER2 antibody-drug conjugate in HER2-positive metastatic breast cancer has already fast-tracked a phase III trial.Trastuzumab-DM1 (T-DM1) has demonstrated comparable results to standard treatment but with much less grade 3-4 toxicity in phase II trial results.

Experimental anti-HER2 antibody-drug conjugate shows 'very good' antitumor activity.
Impressive results from an ongoing study of an anti-HER2 antibody-drug conjugate in HER2-positive metastatic breast cancer has already fast-tracked a phase III trial.
Trastuzumab-DM1 (T-DM1) has demonstrated comparable results to standard treatment but with much less grade 3-4 toxicity in phase II trial results.
"This is the first presentation of an anti-HER2 antibody-drug conjugate used as first-line therapy for patients with advanced breast cancer, and we are encouraged by the results," said lead investigator Edith A. Perez, MD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla. "The study demonstrates that T-DM1 has very good antitumor activity as well as much lower toxicity when evaluated side-by-side with the older standard," specifically trastuzumab (Herceptin) plus docetaxel (Taxotere).
T-DM1 is an experimental agent that combines the anti-HER2 properties of the antibody trastuzumab with targeted delivery of the antimicrotubule agent DM1.
The TDM4450G study randomized 67 women to receive T-DM1 (3.6 mg/kg intravenously every three weeks) and 70 women to receive standard treatment with trastuzumab (6 mg/kg intravenously; 8 mg/kg in cycle 1) plus docetaxel (75 or 100 mg/m2 intravenously on day 1 every three weeks). The women underwent their respective treatments until disease progression or unacceptable toxicity occurred (ESMO 2010 abstract LBA3).
In the T-DM1 group, 13 women (median age, 55) had received prior treatment with trastuzumab. In the standard therapy group, 18 women (median age, 52) had received prior treatment.
After a median of approximately six months of follow up, the researchers found an overall response rate of 48% in patients receiving T-DM1 compared with 41% in patients receiving trastuzumab plus docetaxel. Three women (4.5%) in the T-DM1 group had a complete response, compared with one woman (1.4%) in the trastuzumab plus docetaxel group. The clinical benefit rate, which was calculated as complete response plus partial response plus stable disease rate, was similar in both treatment groups: 55% in the T-DM1 group and 57% in the standard treatment group.
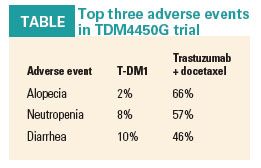
The rate of clinically relevant adverse events was significantly lower in the T-DMI group: 37% vs 75% in the trastuzumab plus docetaxel group. The three most common adverse events of any grade were significantly lower in the experimental arm (see Table). There was one case of congestive cardiac failure in the T-DM1 arm of the study. Autopsy results reported that this event was related to disease progression, Dr. Perez said.
A larger phase III trial is now underway, she added. "This trial is named MARIANNE, and it evaluates taxane plus trastuzumab against T-DM1 as administered in this study, with a third option being T-DM1 plus pertuzumab, another novel anti-HER2 agent," Dr. Perez said.
Giredestrant Combo Yields Positive PFS in Subgroups After CDK4/6i in ER+/HER2– Breast Cancer
December 13th 2025“The magnitude of clinical benefit was clinically meaningful and consistent, and was regardless of PIK3CA mutations or alterations in the PIK3CA pathway, duration of prior CDK4/6 inhibitors, including patients who progress within 6 to 12 months, and the choice of prior CDK4/6 inhibitors,” said Hope S. Rugo, MD.