Treatment-Induced Amenorrhea Remains Controversial in Premenopausal Breast Cancer
SAN FRANCISCO-The impact of achieving amenorrhea during treatment for premenopausal breast cancer is controversial, according to data from the National Cancer Institute of Canada (NCIC) Clinical Trials Group (CTG). A common occurrence among premenopausal breast cancer patients, treatment-induced amenorrhea is often considered a positive prognostic factor. The NCIC CTG data was unable to demonstrate such an effect.
SAN FRANCISCOThe impact of achieving amenorrhea during treatment for premenopausal breast cancer is controversial, according to data from the National Cancer Institute of Canada (NCIC) Clinical Trials Group (CTG). A common occurrence among premenopausal breast cancer patients, treatment-induced amenorrhea is often considered a positive prognostic factor. The NCIC CTG data was unable to demonstrate such an effect.
"Amenorrhea during chemotherapy appeared to have no effect on disease- free or overall survival in our study," Wendy Parulekar, MD, of Queens University in Kingston, Ontario, reported. "Surprisingly, the incidence of amenorrhea was higher with cyclophosphamide (Cytoxan)/epirubicin (Ellence)/fluorouracil (CEF) than with cyclophosphamide/methotrexate/fluorouracil (CMF) despite a lower cumulative dose of cyclophosphamide," she noted. "In receptor-positive patients, amenorrhea appeared to have a beneficial effect on disease-free survival, but this disappeared after controlling for treatment, nodal status, and age. Overall survival appeared unaffected in this group of patients using unstratified and stratified analyses."
Prognostic Value Analyzed
The NCIC investigators studied the incidence and prognostic value of drug- induced amenorrhea (DIA) during adjuvant therapy with anthracycline-containing regimens compared to standard CMF. They analyzed the database of a randomized phase III NCIC CTG trial conducted in 1989 to 1993 that included premenopausal and perimenopausal patients with node-positive breast cancer. Patients had been randomized either to:
CMFcyclophosphamide 100 mg/m2 po days 1 to 14, methotrexate 40 mg/m2 IV days 1 and 8, fluorouracil 600 mg/m2 IV days 1 and 8; or to
CEFcyclophosphamide 75 mg/m2 po days 1-14, epirubicin 60 mg/m2 IV days 1 and 8, and fluorouracil 500 mg/m2 IV days 1 and 8.
Treatment was given every 28 days for six cycles. According to Dr. Parulekar, 541 of the 716 patients in the clinical trial had normal menstruation at randomization and had received six cycles of chemotherapy. Data from these patients were analyzed for the amenorrhea study.
DIA was defined as cessation of menses for 3 or more months during treatment. Complete menstrual data during treatment was available for 473 patients234 (89%) treated with CEF and 239 (86%) treated with CMF. Median ages for both groups were similar: 43.5 years for the CEF group and 43.9 years for the CMF group. Median follow-up for this analysis was 7.7 years.
CEF vs CMF Arm
The incidence of DIA was significantly higher in the CEF arm compared to the CMF arm. (See Table).
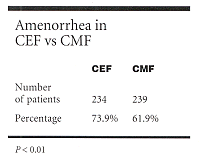
"The incidence of amenorrhea was higher with CEF in all subgroups and was statistically significant in the subgroup of women younger than age 35," Dr. Parulekar said.
DIA did not appear to affect relapse free survival (RFS). Dr. Parulekar reported that the relative risk (RR) was 0.87 (95% CI = 0.66-1.14) in patients with amenorrhea vs patients without amenorrhea (P = 0.3 by log rank).
Adjusting for the variables of T stage, node status, age, receptor status, and treatment using the stratified log rank test demonstrated no effect on RFS (P = 0.8).
Amenorrhea appeared to have no effect on overall survival (OS). In patients with estrogen or progesterone receptor positive tumors (n = 287), onset of amenorrhea affected RFS in univariate analysis only.
During the discussion period, Dr. Parulekar pointed out that no tamoxifen (Nolvadex) was not allowed in this study. The authors of this study agreed that the ability of many of these trials to examine the impact of amenorrhea is poor. Meta-analytic techniques combining data from a number of trials or a prospective interventional study designed and powered to answer this specific question would be the best way to approach this important issue.
Leuprolide Can Prevent Treatment-Related Amenorrhea
"Leuprolide plus adjuvant chemotherapy may preserve ovarian function in selected breast cancer patients," reported Kevin R. Fox, MD, of the University of Pennsylvania Cancer Center in Philadelphia. Younger women with breast cancer are often concerned about maintaining fertility, he noted.
Permanent amenorrhea is the most common irreversible toxicity in women given adjuvant chemotherapy for breast cancer. The incidence is 40% overall and more than 80% in women over age 40 treated with CMF.
Loss of ovarian function is caused by direct toxicity to developing follicles. Dr. Fox and colleagues reasoned that treatment with leuprolide might prevent this problem by suppressing follicle growth during the time chemotherapy was being given.
"This study was done specifically for women wanting to maintain fertility," Dr. Fox said. "All patients had operable metastatic breast cancer. Adjuvant chemotherapy was given with curative intent."
Temporary Amenorrhea
Thirteen patients diagnosed between 1994 and 1999 were given leuprolide during the course of adjuvant therapy as a means of inducing temporary amenorrhea and thus protecting the ovaries from the cytotoxic effects of chemotherapy. Patients ranged in age from 26 to 39 years (median age 35). Five had node-negative disease, and eight had node-positive disease.
Six patients received standard Adriamycin (doxorubicin)/cyclophosphamide (AC) for four cycles. Five received AC followed by paclitaxel (Taxol) for four cycles. One received cyclophosphamide/Adriamycin/fluorouracil (CAF) for 6 months, and one received doxorubicin/docetaxel (Taxotere) followed by CMF. Median follow-up time was 36 months.
The primary study endpoint was resumption of regular menstrual cycling after chemotherapy. Secondary endpoints were relapse and survival rates. Dr. Fox reported on the first 13 patients treated with leuprolide. Minimum follow-up was 1 year.
In 11 patients, depot leuprolide was given in a dose of 3.75-mg IM 1 week prior to chemotherapy and repeated every 3 to 4 weeks until completion of chemotherapy. Two patients received leuprolide 7.5mg on a similar schedule.
Dr. Fox said that all patients became amenorrheic by the second cycle of chemotherapy. Menstrual periods resumed in all leuprolide-treated patients within 12 months of the completion of chemotherapy, with a mean time to recovery of 4.9 months (range 2 to 12 months).
Regular Cycles Reported
Dr. Fox said that 12 of 13 patients reported regular cycles, One reported irregular menses at 16 months from completion of treatment. Four patients also taking tamoxifen continue regular menses. "Estradiol and follicle stimulating hormone (FSH) levels are within premenopausal limits," Dr. Fox reported.
Three patients developed metastatic disease at 19 and 36 months after diagnosis.
Dr. Fox said that while this pilot study provides evidence that leuprolide can be used to maintain menstrual function in women treated with chemotherapy, questions remain about when leuprolide should be given for optimal protective effect. Patient selection also needs clarification.
"Patients treated with AC alone have a fairly low risk of irreversible amenorrhea, but we have seen a 60% rate in patients under age 45 treated with AC plus paclitaxel," he said. The role of leuprolide in receptor-positive patients also needs further study.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.