Tricom Vaccine Delivered Safely With Smallpox, Avipox Vectors
MIAMI BEACH-Sequential Tri-com vaccines against cancers testing positive for carcinoembryonic antigen (CEA) have produced one clinical response and no toxicity among 23 patients in a phase I clinical trial.
MIAMI BEACHSequential Tri-com vaccines against cancers testing positive for carcinoembryonic antigen (CEA) have produced one clinical response and no toxicity among 23 patients in a phase I clinical trial.
John L. Marshall, MD, director of Developmental Therapeutics and Gastrointestinal Oncology, Lombardi Cancer Center, Washington, DC, presented preliminary results (abstract 798) at Molecular Targets and Cancer Therapeutics, an international conference cosponsored by the American Association for Cancer Research (AACR), National Cancer Institute (NCI), and European Organization for Research and Treatment of Cancer (EORTC).
"We’ve shown so far that it is safethe patients handled it extremely welland there’s evidence of clinical activity in one patient with small-cell lung cancer (SCLC) following just two vaccinations," said Dr. Marshall, associate professor of medicine, Georgetown University School of Medicine. He described the Tricom vaccine as "arguably one of the most potent anticancer vaccines available."
As its name suggests, Tricom contains genes for three moleculesB7-1, ICAM, and LFA-3that have been shown in preclinical testing to enhance antigen-specific immune response. The vaccine is attached to a live virus that has been spliced with a CEA gene in order to improve T-cell activation against the CEA antigen.
The phase I trial was carried out in three phases. In the first phase, patients received all doses of the vaccine in a vector of recombinant avipox, a fowlpox virus that infects birds. For the second phase, the first inoculation was delivered in recombinant vaccinia, a smallpox vaccine, with the avipox vector reserved for delivery of booster doses. In the third phase, patients received the sequence of vaccinia and avipox vectors plus granulocyte macrophage-colony stimulating factor (GM-CSF), another stimulator of immune response.
"Vaccinia is the smallpox vaccine in essence. It has been genetically engineered to carry genes for the immune response," Dr. Marshall said. All of the patients in the preliminary group had been inoculated previously against smallpox, but the trial’s protocol has recently been amended to include people who have not previously been exposed to the smallpox virus.
So far, he said, there has been no evidence to suggest that cancer patients have weakened immune systems that could make them more vulnerable to smallpox. "Our vaccine trial clearly shows patients can mount a significant immune response when guided," he said.
Starting with three-patient cohorts in the avipox-only arm, the investigators have given the vaccine in escalating doses at 28-day intervals. The protocol uses a needle-free injection system to deliver the vaccine subcutaneously. "It is essentially a CO2-powered injection; a high-pressure stream of vaccine is delivered through the skin," Dr. Marshall told ONI. "Theoretically, that disperses the vaccine better."
All of the patients in the study had incurable CEA-positive cancers. Most had colon cancer, he said, but lung, breast, stomach, and pancreatic cancers were also represented in the trial.
One patient with lung cancer had a normal PET scan after receiving two doses of Tricom in the avipox vector, Dr. Marshall said. This patient has been in the study since March 2001 and is still being treated. A colon cancer patient in an earlier study also had a complete response, he said. After 15 months, her cancer started to progress again, but not at the original sites, and her CEA had not gone up.
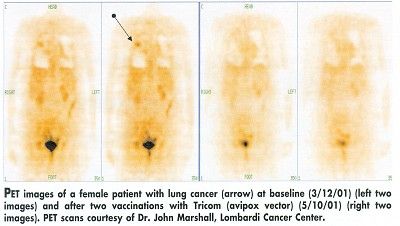
Dr. Marshall said he hoped to have data comparing the efficiency of all three arms by early 2002 and to evaluate the addition of interleukin-2 (IL-2) in future testing. A randomized phase II trial is being planned, he said.
While Dr. Marshall predicted that the vaccine has potential for broad use as an adjuvant treatment, especially when cancers recur, he also said he did not want to discount it as a possible first-line cancer therapy.
At the American Society for Clinical Oncology (ASCO) annual meeting in May, he had reported that patients with a higher immune response to the CEA vaccine lived longer than those who had little or no immune response. "We’re trying to take advantage of all we have learned to date," he said.