Tykerb Approved for Metastatic HER2+ Breast Cancer
Tykerb (lapatinib, GlaxoSmithKline) has received US Food and Drug Administration approval in combination with Xeloda (capecitabine, Roche) for the treatment of locally advanced or metastatic breast cancer in patients whose tumors over-express the HER2 receptor and who have previously received other cancer drugs, including an anthracycline, a taxane, and trastuzumab (Herceptin).
ROCKVILLE, MarylandTykerb (lapatinib, GlaxoSmithKline) has received US Food and Drug Administration approval in combination with Xeloda (capecitabine, Roche) for the treatment of locally advanced or metastatic breast cancer in patients whose tumors over-express the HER2 receptor and who have previously received other cancer drugs, including an anthracycline, a taxane, and trastuzumab (Herceptin). Tykerb is the first targeted, once-a-day oral treatment authorized for this indication.
FDA approved the drug after a priority review of a randomized, phase III trial, which demonstrated that patients treated with Tykerb and Xeloda had a significant improvement in time to progression (TTP) and a higher response rate than patients who received Xeloda alone.
"Tykerb is a significant breakthrough for women with advanced HER-positive breast cancer," said Paolo Paoletti, MD, senior vice president of the company's Oncology Medicine Development Center. The approval reflects more than 16 years of research by the company, including more than 60 clinical trials and investigator-initiated collaborative research studies. "We are dedicated to the further study and development of Tykerb in a variety of settings, including adjuvant breast cancer as well as in other solid tumor types," Dr. Paoletti said.
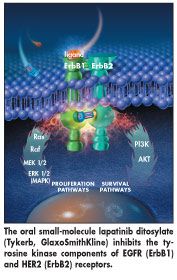
Tykerb, a new molecular entity, enters the cell and uses discrete, multiple pathways to target the tyrosine kinase components of the EGRF and HER2 receptors on cells (see Figure). Over-expression of the receptors is associated with cell proliferation and other processes related to tumor progression, invasion, and metastases, and often indicates a poor prognosis and reduced overall survival.
Pivotal Trial
The international, multicenter pivotal trial submitted by GlaxoSmithKline enrolled 399 HER2-positive, previously treated women with locally advanced or metastatic breast cancer. The combination drug group (n = 198) received five 250 mg Tykerb tablets at one time on each day of a 21-day cycle, as well as Xeloda 1,000 mg/m2 bid on days 1 through 14 of each cycle. The Xeloda-only arm (n = 201) received a dose of 1,250 mg/m2 bid for the first 2 weeks of the 3-week cycle.
At the recommendation of the trial's Independent Data Monitoring Committee, GlaxoSmithKline halted enrollment early in 2006 after a preplanned interim analysis of 321 patients showed the combined treatment group had a superior TTP, compared with the Xeloda-only arm. This statistically significant result exceeded the primary endpoint.
Updated Analysis
Researchers continued to follow all women in the study, and the trial data submitted to FDA contained separate updated analyses of all 399 participants made by the monitoring committee and the trial investigators 4 months after the interim analysis.
The monitoring committee's analysis reported 82 time-to-progression events for the Tykerb plus Xeloda patients and 102 for the Xeloda-only patients. The investigator's analysis reported 121 and 126 such events, respectively.
Both analyses showed a significant difference between the two groups in time to progression. The monitoring committee reported a median of 27.1 weeks for the drug-combination arm and 18.6 weeks for the Xeloda-only group (HR 0.57; P = .00013), for a 43% reduction in risk. The investigators reported similar results: 23.9 weeks and 18.3 weeks, respectively (P = .00762).
The monitoring committee reported a response rate of 23.7% for the drug combination vs 13.9% for Xeloda alone, and investigators put the response rates at 31.8% and 17.4%, respectively.
At the time of the updated analyses, there were 55 deaths (28%) among the Tykerb/Xeloda patients and 64 (32%) among those receiving Xeloda alone. Data for survival analysis have not matured.
Adverse Events
The most common adverse events in the Tykerb/Xeloda arm included diarrhea (65% vs 40% in the comparison group), hand-foot syndrome (53% vs 51%), nausea (44% vs 43%), rash (28% vs 14%), and vomiting (26% vs 21%). The most common grade 3 events were diarrhea (13% vs 10%, respectively) and hand-foot syndrome (12% vs 14%). One grade 4 diarrhea occurred in the Tykerb/Xeloda arm; no grade 4 events were reported among the Xeloda-only patients.
Adverse events leading to therapy discontinuation were similar in the two arms (14%). However, among the Tykerb-treated patients, three developed a grade 2 and one had a grade 3 decrease in left ventricular ejection fraction (LVEF).
"Left ventricular ejection fraction should be evaluated in all patients prior to initiation of treatment with Tykerb to ensure that the patient has a baseline LVEF that is within the institution's normal limits," the product labeling warns. "LVEF should continue to be evaluated during treatment with Tykerb to ensure that LVEF does not decline below the institution's normal limits."