Use of Strontium-89 in Metastatic Cancer: US and UK Experience
The utility of strontium-89 (Sr-89) in thetreatment of advanced metastatic prostate cancer has been examinedin numerous clinical trials. Early phase II efficacy studies demonstratedthat the majority of Sr-89-treated patients
The utility of strontium-89 (Sr-89) in the treatment of advanced metastatic prostate cancer has been examined in numerous clinical trials. Early phase II efficacy studies demonstrated that the majority of Sr-89-treated patients experienced a decrease in bone pain and analgesia requirements, with overall response rates of 75% to 80%. Toxicity was mainly hematological and clinically insignificant, suggesting that 40 mCi/kg (1.5 MBq/kg) is an optimal palliative Sr-89 dose. A subsequent study verified that the observed clinical response is due to radioactive strontium and is not a chemical effect. Two more recent phase III studies have extended these early results. The TransCanadian study showed that concomitant Sr-89 significantly reduced the appearance of new painful metastases, analgesic requirements, and serum levels of tumor markers compared with radiotherapy alone. Overall, these studies support Sr-89 as an effective systemic radiopharmaceutical for the palliation of bony metastases.
Introduction
Although many primary malignancies cause metastases, the most important among American males is prostate cancer, with a rate of bone metastasis of at least 50% [1]. The survival time for patients with metastatic disease is approximately 2 years. During this time, patient management must include the treatment of symptoms related to painful bone lesions [2]. Aside from pain, late stage patients are compromised by pathologic fractures, neurologic deficits, decreased mobility, and the associated depression and anxiety that may accompany these physical symptoms. The main goals of treatment are pain palliation and improvement in quality of life. These are also among the major criteria used to evaluate the effectiveness of therapeutic regimens used in stage D prostatic carcinoma.
The management of bone metastases is a difficult problem--therapy is not curative and there is no real consensus among investigators concerning the best therapeutic strategies [2]. Treatment options include conventional analgesics, hormonal manipulation, external-beam radiation, chemotherapy, and radiopharmaceutical therapy [3]. The clinician's challenge in choosing the appropriate therapy for a given patient is to weigh the benefits of a treatment against associated adverse effects.
Since multiple sites of pain are the main therapeutic concern, treatment with narcotic analgesics has traditionally been indicated for bone metastasis [1,3]. However, opiates are less than ideal due to adverse gastrointestinal and sedative effects. Hormonal therapy is another common therapeutic choice for advanced prostate cancer since these tumors are initially androgen dependent. Although an effective first-line management in approximately 70% of prostate cancer patients, androgen deprivation is associated with a high rate of relapse accompanied by disease progression. Second- and third-line hormonal treatment may be appropriate in 30% of these patients, but the benefits are often short-lived. Chemotherapy is considered to provide few benefits at this stage of the disease.
Radiation Therapy
An alternative first-line treatment option is radiotherapy [1]. Although effective for locally confined lesions, local radiation therapy is inadequate for the treatment of multiple metastatic sites [4]. Hemibody irradiation (HBI) is more effective in the latter case, with several studies demonstrating that it provides a palliative response rate of 75% [5]. However, HBI is associated with significant adverse effects such as bone marrow suppression, nausea, vomiting and lung toxicity.
Radiopharmaceutical therapy has recently become a primary treatment for advanced metastatic prostate cancer [6]. The administration of phosphorus-32 (P-32) was first introduced in humans in 1942 for the treatment of metastatic bone cancer [7]. Since that time, attempts to develop this isotope as a radiopharmaceutical have produced efficacy rates of 60% to 90% [6]. However, P-32 is not selectively deposited in bone tissue. Instead, the isotope is taken up by a variety of other tissues and systemic administration results in severe myelosuppression that limits its clinical usefulness.
Strontium-89--The limitations of P-32 created a clinical need for a radiopharmaceutical agent that relieves pain and improves the quality of life for patients with advanced prostate cancer. Systemic delivery of radiation by an isotope selective for bone tissue would maximize efficacy and minimize toxicity. Strontium-89 (Sr-89, Metastron) is such an isotope with several other desirable characteristics that increase its clinical utility.
Chemically, Sr-89 is similar to calcium and is preferentially taken up in osteoblastic tissue while the unabsorbed isotope is excreted in the urine the first 2 to 3 days following injection [1,6]. The tumor-to-marrow absorbed dose ratio for Sr-89 is greater than 10:1, suggesting that patients would experience minimal myelosuppression. This is indeed what is observed clinically. In addition, Sr-89 is a pure beta-emitter with a maximum beta energy of 1.46 MeV and a longer half-life (50.5 days) than P-32 (14.3 days), characteristics that result in minimal irradiation of healthy tissues and a duration of at least 100 days in osteoblastic target sites. These properties provide the rationale for Sr-89 as a therapeutic isotope.
Strontium-89 Clinical Trials
Early Efficacy Trials
TABLE 1

Clinical Trials of Strontium-89 in Patients With Metastatic Bone Disease
The clinical utility of Sr-89 was shown in a series of trials that evaluated its role in the management of patients with advanced prostate cancer. Phase II trials were initiated in both England and the United States with encouraging results reported by both groups. These two early studies, along with later Phase III trials, are summarized in Table 1.
The first of these trials was designed to address the efficacy and safety of Sr-89 treatment [8]. The report summarized data on 202 patients with bone pain from metastatic disease who were treated with Sr-89. The first 20 patients received an intravenous (IV) Sr-89 dose of 30 mCi/kg (1.11 MBq/kg), while the remaining 182 patients received 40 mCi/kg (1.48MBq/kg) as the initial dose. Patients were monitored during the 3-month study by pain and medication diaries, sleep patterns, bone scans, and a Karnofsky Index. The majority of the surviving patients (100) had prostate cancer, while 28 patients had breast cancer and nine patients had other carcinomas.
The results demonstrated that most patients experienced a decrease in bone pain and analgesia with an overall response rate of 80% [8]. The reduced pain level typically occurred 2 to 3 weeks post-injection. Twenty percent of patients had no hematologic toxicity, while the remainder experienced mild hematological depression that consisted of a 15% to 20% decrease in total platelet and white blood cell (WBC) counts.
Kinetic analysis of whole body profiles revealed that 88% of the Sr-89 dose was retained for at least 100 days in one patient with widespread metastases. In addition, Sr-89 retention was observed primarily at metastatic sites, contributing to the beneficial clinical responses.
Based on these clinical findings, Robinson et al recommended Sr-89 therapy for patients with metastatic prostate cancer who fail hormonal treatment. They further concluded that a systemic dose of 40 mCi/kg (1.48 MBq/kg) is safe and effective for the treatment of painful osseous metastases in these patients.
Multicenter Study--The clinical experience of Robinson et al was confirmed by the multi-center study of Sr-89 conducted by Laing and colleagues [9]. This study evaluated 83 patients with prostatic carcinoma who failed conventional therapy. Similar to Robinson et al, these investigators found that an Sr-89 dose of 1.5 MBq/kg provided meaningful clinical response to 75% of patients, with 22% becoming pain free. Although the observed mean percentage drop in platelet levels was significantly related (P less than 0.02) to increasing Sr-89 doses (0.7 to 3.0 MBq/kg), the effect was deemed mild and reversible.
It should be noted that, although not included in the efficacy analyses, both studies documented pain relief in some patients who did not survive the three month treatment period [8,9]. Therefore, Sr-89 response rates found in practice may be higher than values reported in these controlled studies. Clearly, the two independent clinical studies reviewed above both demonstrate a therapeutic potential of Sr-89 therapy for the management of metastatic prostate disease.
These early findings were updated by Robinson and colleagues in 1993 [1]. At present, 15 years of accumulated data continues to demonstrate that Sr-89 provides pain relief and improved functional status in 80% of patients with bone metastases with minimal adverse effects.
Chemical vs Radiation Effects of Strontium--The studies reviewed above provided extremely encouraging results for the palliative effects of Sr-89 in metastatic bone disease. However, they led to the question of whether the observed effects were actually attributable to radioactive strontium. To address this, Lewington et al carried out a study to determine the contribution of any chemical effects of strontium in pain palliation of patients with advanced prostate cancer [10].
FIGURE 1

Clinical response to Sr-89 or elemental strontium (placebo) at 5 weeks post injection in patients with metastatic prostate cancer
Thirty-two patients who failed conventional treatments participated in a randomized, double-blind crossover study comparing Sr-89 with stable strontium as the placebo [10]. Patients received either 50 mg stable Sr chloride (ie, Sr-88) or 150 MBq Sr-89 chloride containing 50 mg stable Sr chloride. Each treatment was spiked with a tracer dose of Sr-85 to insure proper blinding. Patient responses were evaluated by numerical grading of general condition, mobility, analgesics, pain, and overall assessment. Patients were crossed-over to the alternative protocol at 6 weeks post-injection.
The results showed that only those patients receiving Sr-89 reported dramatic improvement, while the majority of patients treated with elemental strontium reported no significant change or deteriorated (P less than 0.01, Figure 1) [10]. In terms of toxicities, differences in platelet counts were documented between the two groups, with Sr-89 treatment resulting in a 25% mean reduction of baseline values that was not clinically significant. Lewington et al concluded that the placebo effect is limited, while there is a clear therapeutic response to Sr-89 with no immediate adverse reactions or significant toxicities.
Phase III Clinical Trials
The previously described studies have provided clinical evidence supporting the effectiveness and safety of Sr-89 as a palliative therapy for patients with metastatic prostate disease. This led to the execution of two major complementary Phase III clinical trials. One consisted of a series of studies in the United Kingdom (UK) (1988-1991) that directly compared Sr-89 with external beam radiotherapy [11]. The second trial, referred to as the TransCanadian study, attempted to determine the potential of Sr-89 as an adjuvant to radiotherapy [12].
Sr-89 vs External Beam Radiotherapy--The comparative UK studies involved 305 patients classified by the investigator as eligible for either local radiotherapy (n = 148) or HBI (n = 157) [11]. Patients were randomized to receive either external beam irradiation (20 Gy in five daily fractions or a single fraction of 8 Gy, local radiotherapy; single fractions of 6 Gy for upper or 8 Gy for lower HBI) or Sr-89 (200 MBq [5.4 mCi]). Patients were monitored for original and new sites of pain, analgesic intake, and toxicity and the data was summarized at 3 months post-treatment.
FIGURE 2

Sr-89 vs Local Radiation Therapy
Dramatic pain improvement at the original site was reported by 44.1% of patients after Sr-89 and 36.4% after local radiotherapy (Figure 2) [11]. Importantly, it was further observed that 65% of Sr-89 treated patients were free of new pain sites compared with 46.5% of patients treated with local radiotherapy
(P less than 0.01). This resulted in the added benefit of a significantly lower requirement for additional radiotherapy in the former compared with the latter group (P less than 0.01).
Similar results were found in comparison of Sr-89 and hemibody radiotherapy [11]. At 3 months, pain improvement at the original sites was reported by 66.1% of patients receiving Sr-89 and 63.6% of HBI-treated patients. However, the radio-pharmaceutical resulted in a significantly larger proportion of patients who were free of new pain sites (73.3%) compared with those receiving HBI (54.6%, P less than 0.05).
An important advantage to Sr-89 radiotherapy that was found in this trial was a lower incidence of adverse effects [11]. Gastrointestinal effects were reported by 42.9% of patients after HBI, 27.3% of patients in the local radiotherapy group, and only 10.1% of patients receiving Sr-89. The common adverse events encountered with Sr-89 treatment were hematologic, with a depression of platelet and leukocytes. However, as in earlier trials, this was not considered to be clinically relevant in most cases.
The reduction in the development of new pain sites with Sr-89 is an exciting clinical finding, suggesting disease stabilization. It should be noted that, although there was a trend towards improved patient survival in the Sr-89 group (33 weeks), the result was not significantly different (P = 0.11) from external beam radiotherapy (28 weeks) [11]. However, the data do clearly indicate that Sr-89 offers several advantages over irradiation including an improved quality of life for the patient and less of a drain on hospital resources.
Sr-89 Adjunctive to Radiotherapy--The TransCanandian study was designed to evaluate the efficacy of Sr-89 adjuvant to local external beam radiation in the management of metastatic prostate cancer [12]. This trial involved 126 patients with hormonal resistant disease and adequate hematologic function. Local field irradiation was typically dosed at 30 Gy (10 fractions over 14 days or 20 Gy in five fractions over 7 days) with the exception of rib lesions that required single fractions of up to 10 Gy. Placebo or IV Sr-89 (10.8 mCi) was administered with 7 days of radiotherapy. Patients were assessed for survival, pain relief and progression, analgesic intake, need for additional radiotherapy, serum tumor markers, quality of life, and toxicities.
FIGURE 3

New Sites of Pain
The results showed that the rate of complete pain relief ranged from 30% to 60% and was similar in both the placebo and Sr-89 groups [12]. Although adjuvant use of Sr-89 over local field radiation alone demonstrated a consistent trend of improved overall pain relief, this finding was not statistically significant. Important from the patient's point of view, Sr-89 treatment resulted in a significant reduction in the appearance of new painful metastases compared with radiotherapy alone (P less than 0.002, Figure 3). In terms of analgesia requirements, a greater proportion of patients receiving adjuvant Sr-89 reduced their analgesic intake by at least 50% or eliminated analgesic intake entirely (P less than 0.05) compared with the placebo group.
FIGURE 4
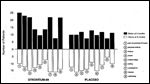
Quality of Life
Quality of life is also major concern in the management of this patient population and was evaluated with an extensive questionnaire encompassing nine categories and a supplemental section concerning pain. The Sr-89 group reported a significantly improved overall quality of life (P = 0.006), including particular improvements in physical activity and alleviation of pain (P less than 0.05) compared with those receiving radiotherapy alone (Figure 4).
In addition to improvements in clinical disease parameters, Sr-89 treatment resulted in reductions of tumor markers, suggesting a tumoricidal effect [12]. It was observed that more patients receiving Sr-89 experienced a drop in prostatic acid phosphatase (PAP), prostatic specific antigen (PSA, P less than 0.01) and alkaline phosphatase (P less than 0.01) compared with placebo during the first four study months.
Future Clinical Challenges
Sr-89 Dose Optimization--As more clinicians move towards treating patients with Sr-89, optimization of dosing, amelioration of toxicity, and augmentation of the therapeutic response are areas to be explored. Imminent among these is the question of the usefulness of Sr-89 dose fractionation. RTOG 8821 is a preliminary study designed to address this issue (Porter et al, unpublished). Patients were randomized to receive a 4 mCi Sr-89 with subsequent dose escalation to 6.5, 9 and 11.5 mCi. Hematological toxicities were used as a criteria for evaluating dosing and it was determined that 4-6.5 mCi is efficacious and produces minimal adverse effects (unpublished observations).
While it does appear that a dose response relationship exists between Sr-89 and complete pain relief, the optimal dosage has not yet been definitively established [13]. In addition, the benefits, if any, of Sr-89 dose fractionation need to be determined.
Enhancement of Sr-89 Therapeutic Response--Enhanced responses to irradiation have been observed with concurrent cisplatin infusion in murine models [14]. This has prompted a phase I/II trial of combination Sr-89/cisplatin therapy in patients with hormone refractory metastatic prostate cancer. Encouraging preliminary data have been reported suggesting good pain relief, reduced analgesic requirements, decreased serum tumor markers, and only mild toxicity. Further evaluation of the effect of Sr-89 sensitization by cisplatin are warranted.
In summary, the clinical data presented in this review clearly advocate a significant role for Sr-89 as a palliative treatment for patients with hormone refractory metastatic prostatic carcinoma. The availability of this agent will allow clinicians further flexibility in the development of novel treatment paradigms in the management of this difficult, progressive neoplasm.
References:
1. Robinson RG, Preston DF, Baxter, KG et al: Clinical experience with Sr-89 in prostatic and breast ca patients. Semin Oncol 20:44-48, 1993.
2. Nielsen OS, Munro AJ, Tannock IF: Bone metastases: Pathophysiology and management policy. J Clin Oncol 9:509-524, 1991.
3. McEwan AJB, Porter AT, Venner PM, et al: An evaluation of the safety and efficacy of treatment with strontium-89 in patients who have previously received wide field radiotherapy. Antibody Immunoconj Radiopharmac 3:91-96, 1990.
4. Porter AT, McEwan AJB: Sr-89 as an adjuvant to external beam radiation improves pain relief and delays disease progression in advanced prostate cancer. Semin Oncol 20:38-43, 1993.
5. Hoskin PJ: Scientific and clinical aspects of radiotherapy in the relief of bone pain. Cancer Surveys 7:69-86, 1988.
6. Robinson RG: Strontium-89 for bone pain due to blastic metastatic disease. Appl Radiol August:44-47, 1993.
7. Friedell HL, Storaasli JP: The use of radioactive phosphorus in the treatment of carcinoma of the breast with widespread metastases to bone. Am J Roentgenol Radium Ther 64:559-75, 1950.
8. Robinson RG, Blake GM, Preston DF, et al: Strontium-89: treatment results and kinetics in patients with painful metastatic prostate and breast cancer in bone. RadioGraphics. 9:271-281, 1989.
9. Laing AH, Ackery DM, Bayly RJ, et al: Sr-89 chloride for pain palliation in prostatic skeletal malignancy. Br J Radiol 64:816-822, 1991.
10. Lewington VJ, McEwan AJ, Ackery DM, et al: A prospective, randomized double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer 27:954-958, 1991.
11. Bolger JJ, Dearnaley DP, Kirk D, et al: Strontium-89 (Metastron) versus external beam radiotherapy in patients with painful bone metastases secondary to prostatic cancer. Semin Oncol 20:32-33, 1993.
12. Porter AT, McEwan AJB, Powe JE, et al: Results of a randomized phase-III trial to evaluate the efficacy strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. J Rad Oncol 25;805-813, 1993.
13. Mertens WC, Stitt L, Porter AT: Strontium 89 therapy and relief of pain in patients with prostatic ca metastatic to bone: A dose response relationship? Am J Clin Oncol 16:238-242, 1993.
14. Mertens WC, Porter AT, Reid RH, et al: Strontium-89 and low-dose infusion of cisplatin for patients with hormone refractory prostate carcinoma metastatic to bone: A preliminary report. J Nuclear Med 33:1437-1443, 1992.