USPSTF Recommends Low-Dose CT Screening for Heavy Smokers
The USPSTF published its final recommendation on screening for lung cancer on December 31, concluding that all people between the ages of 55 and 80 years who are at high risk for lung cancer should undergo low-dose CT screening.
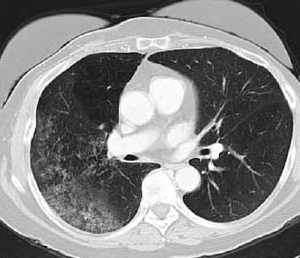
Bronchoalveolar cell carcinoma detected by CT (image provided by the American Thoracic Society).
The US Preventive Services Task Force (USPSTF) published its final recommendation on screening for lung cancer on December 31, concluding that all people between the ages of 55 and 80 years who are at high risk for lung cancer should undergo low-dose computed tomography screening. High-risk is defined as any current heavy smoker or former heavy smoker who has quit within the last 15 years.
“It’s clear that the longer and the more a person smokes, the greater their risk is for developing lung cancer,” said the USPSTF task force’s co-vice chair Michael LeFevre, MD, of the University of Missouri School of Medicine, in a press release.
Lung cancer remains the leading cause of cancer death in the United States, and is the third most common of all cancers. The screening recommendations include smokers with a 30 pack-year smoking history; annual screening in such patients should be discontinued when the 15-year period since quitting has elapsed or if another health problem develops that would limit life expectancy or willingness to have lung surgery.
The new recommendations are based primarily on the results of the National Lung Screening Trial, which enrolled 53,454 individuals between August 2002 and April 2004. It compared low-dose CT screening to chest radiography, and found an overall reduction in death from any cause in the CT group of 6.7% (95% CI, 1.2-13.6; P = .02). There was also a relative reduction in mortality from lung cancer with low-dose CT of 20% (95% CI, 6.8-26.7; P = .004). Results of the NLST were published in the New England Journal of Medicine in 2011.
The harms associated with low-dose CT screening, as the USPSTF recommendations discuss, lie largely in the high rates of false positives. A total of 96.4% of the positive low-dose CT results in the NLST were false positives (24.2% of all screening tests were positive). The USPSTF notes that in a “high-quality screening program, further imaging can resolve most false-positive results.” Some patients, however, will undergo invasive procedures that still do not result in a diagnosis of lung cancer.
In spite of this high false-positive rate, the USPSTF found insufficient evidence on the harms associated with such findings. One modeling study found that between 10% and 12% of screening-detected cancers are overdiagnosed, meaning they would not have been detected in the patient’s lifetime without screening. Still, the benefits of screening have been found to justify such a program in high-risk individuals.
Even with the new recommendation for a screening program, the USPSTF stressed that such a program does not change the primary lung cancer prevention method. “Screening for lung cancer, while beneficial, should not be an alternative to quitting smoking,” said the task force chair Virginia Moyer, MD, MPH, the vice president for maintenance of certification and quality at the American Board of Pediatrics. “The best way to reduce the sickness and death associated with lung cancer is to promote smoking cessation and protect people who are non-smokers from tobacco smoke exposure.”