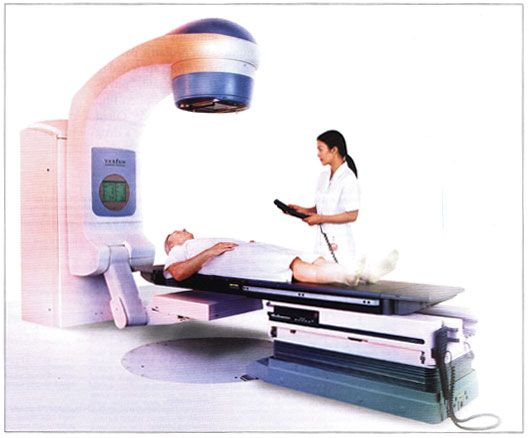
Varian Medical Systems Introduces ‘Acuity’ for Precise Cancer Targeting
PALO ALTO, California-Varian Medical Systems, Inc. has received 510(k) clearance from the FDA for Acuity, a new digital imaging product that integrates planning, simulation, and verification software for treating cancer with radiation therapy. It works with Varian’s RPM Respiratory Gating System to track tumor motion during simulation and verification. It is intended to accelerate adoption of intensity modulated radiation therapy (IMRT). By performing patient set-up and treatment plan verification, Acuity frees the linear accelerator to be used exclusively for treatment delivery, Varian said in a press release announcing the new product.
PALO ALTO, CaliforniaVarian Medical Systems, Inc. has received 510(k) clearance from the FDA for Acuity, a new digital imaging product that integrates planning, simulation, and verification software for treating cancer with radiation therapy. It works with Varian’s RPM Respiratory Gating System to track tumor motion during simulation and verification. It is intended to accelerate adoption of intensity modulated radiation therapy (IMRT). By performing patient set-up and treatment plan verification, Acuity frees the linear accelerator to be used exclusively for treatment delivery, Varian said in a press release announcing the new product.
Acuity pairs a high-resolution x-ray tube and amorphous silicon flat panel image detector to instantly produce and display on a computer workstation high-resolution, distortion-free radiographic and fluoroscopic digital images of patients in their treatment position. These images are used for comparison with treatment planning reference images so that clinicians can quickly see problems and make necessary adjustments. Acuity is also adapted for simulating and supporting delivery of image-guided brachytherapy treatments.
The system is integrated with Varian’s VARiS Vision data management system and Eclipse treatment planning to streamline department workflow. It works with products from other vendors through use of the DICOM-RT standard.