What Path to Approval Has Dasatinib Taken in CML/ALL?
Over the past 4 years, the FDA has accepted a number of NDA submissions for dasatinib, but it has yet to receive approval in CML/ALL.
Over the past 4 years, the FDA has accepted a number of NDA submissions for dasatinib, but it has yet to receive approval in CML/ALL.
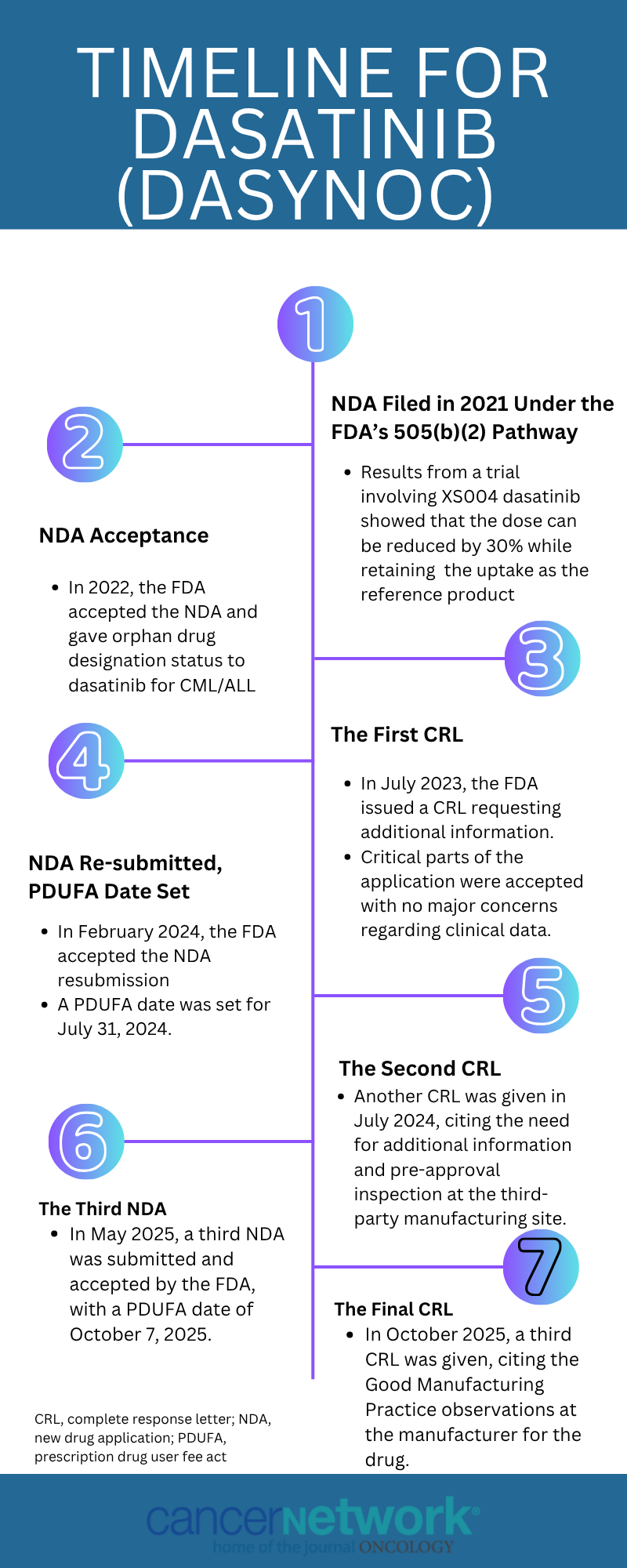
The road to approval has not been straight for amorphous dasatinib (Dasynoc) for patients with chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL). After numerous new drug application (NDA) submissions followed by complete response letters (CRLs), the FDA has once again given the treatment a CRL. Each time, the issue has not related to the clinical data but rather the manufacturing side of the treatment.
Below is a breakdown of the FDA’s actions and decisions beginning in 2021, up until the latest CRL in 2025.
NDA Is Filed
In December 2021, the developer of dasatinib submitted an NDA under the 505(b)(2) regulatory pathway.1 Results from a phase 1 comparative bioavailability study (NCT05439408) were a reference point for this submission. The results showed the dosage could be reduced by 30% compared with reference dasatinib (Sprycel) while maintaining the same uptake as the reference product.2
NDA Acceptance
In January 2022, the FDA announced the acceptance of the NDA for dasatinib.2 In June and November 2022, respectively, the FDA granted orphan drug designation to dasatinib in the CML and ALL disease states.3 The press release from the developers noted that the “product candidate may provide a major contribution to patient treatment due to the gastric pH–resistant qualities of the formulation and the significant frequency of concomitant use of acid-reducing agents in the ALL population.”3
FDA Issues the First CRL
In July 2023, the FDA issued a CRL asking for additional information, specifically for “doctors and users related to [dasatinib] dosing and a third-party manufacturing facility.”4 It was noted that there were no deficiencies with the data.
NDA Resubmitted With PDUFA Date Set
In February 2024, the FDA accepted a resubmitted NDA for dasatinib for patients with CML.5 The Prescription Drug User Fee Act (PDUFA) date was set for July 31, 2024. Upon approval, the company was set to commercially launch the agent on September 1, 2024.
FDA Sends the Second CRL
Ahead of the PDUFA date in July 2024, the FDA gave a second CRL to dasatinib in CML.6 The FDA cited a need for additional information for “labeling comprehension and the preapproval inspection at the third party’s manufacturing site.”6 The inspection had previously occurred from June 10 to 19, 2024. Again, the FDA did not need additional studies or data to support the approval. At the time, developers planned to meet with the manufacturing facility to address the concerns as well as the FDA to discuss the labeling.
The Third NDA Submission
In May 2025, the NDA was resubmitted for dasatinib as a treatment for patients with CML and ALL.7 The FDA had set the PDUFA date for October 7, 2025. Developers were looking to commercially launch the product in October if the PDUFA date was met.
The Third CRL Is Given
In October 2025, the FDA gave a third CRL to dasatinib for patients with CML/ALL.8 The reasoning was based on the Good Manufacturing Practice observations at a contract manufacturer. Although no direct observations were made regarding the product, the FDA decided to pause all approvals from that facility until corrective actions occurred. A meeting with the FDA is scheduled to take place in December 2025. Per Andersson, CEO of Xspray Pharma, noted that developers would “work closely with both the manufacturer and the FDA to expedite the process and enable a resubmission as soon as the corrective actions have been completed.”8
References
- Center for Drug Evaluation and Research: application number: 216099Orig1s000. FDA. December 10, 2021. Accessed October 21, 2025. https://tinyurl.com/3p34asdt
- About us - a timeline. Xspray Pharma. Accessed October 21, 2025. https://tinyurl.com/4xvcy829
- Xspray Pharma’s drug candidate XS004 granted orphan drug designation in the US for the treatment of acute lymphoblastic leukemia. News release. Xspray Pharma. November 30, 2022. Accessed October 21, 2025. https://tinyurl.com/3vz8cavz
- Xspray Pharma receives request for additional information concerning Dasynoc from FDA. News release. Xspray Pharma. July 11, 2023. Accessed October 21, 2025. https://tinyurl.com/6z46zatx
- FDA accepts Xspray Pharma’s NDA-resubmission for Dasynoc – PDUFA date set to 31 July 2024. News release. Xspray Pharma. February 12, 2024. Accessed October 21, 2025. https://tinyurl.com/9spn57dh
- Xspray Pharma shares new information on Dasynoc, a novel CML treatment in development. News release. Xspray Pharma. July 26, 2024. Accessed October 21, 2025. https://tinyurl.com/mvmr48de
- FDA sets PDUFA-date for Xspray Pharma's re-submitted application for Dasynoc. News release. Xspray Pharma. May 14, 2025. Accessed October 21, 2025. https://tinyurl.com/4ck9y989
- Xspray Pharma provides update on the FDA process for Dasynoc - observations at contract manufacturer delay approval. News release. Xspray Pharma. October 8, 2025. Accessed October 21, 2025. https://tinyurl.com/yc366ske