ZD6474 (Zactima) Advances to Phase III Trial in NSCLC
ZD6474 (Zactima), a once-daily oral drug that simultaneously blocks three tumor cell signaling pathways, looked promising in phase II studies vs gefitinib (Iressa) in advanced nonsmall- cell lung cancer (NSCLC) and can be combined with docetaxel (Taxotere), but the decision to move the drug into a phase III clinical trial triggered comments from the discussant (see Vantage Point) at the American Society of Clinical Oncology (ASCO) 42nd Annual Meeting.
ATLANTA--ZD6474 (Zactima), aonce-daily oral drug that simultaneouslyblocks three tumor cell signaling pathways,looked promising in phase II studiesvs gefitinib (Iressa) in advanced non-small-cell lung cancer (NSCLC) and canbe combined with docetaxel (Taxotere),but the decision to move the drug into aphase III clinical trial triggered commentsfrom the discussant (see Vantage Point)at the American Society of Clinical Oncology(ASCO) 42nd Annual Meeting.
Monotherapy Trial
Ronald B. Natale, MD, of Cedars SinaiOutpatient Cancer Center, Los Angeles,reported final results from a two-part,double-blind, randomized phase II trialthat compared ZD6474 to gefitinib inpatients with advanced NSCLC (abstract7000). ZD6474 targets the vascularendothelial growth factor receptor(VEGFR), epithelial growth factor receptor(EGFR), and RET receptor tyrosinekinases. Gefitinib inhibits the EGFRtyrosine kinase. (RET receptor tyrosinekinase activity is involved in the growthof certain thyroid tumors.)
"We are moving from the era of singletargetedagents to those that are multitargeted,testing them first as monotherapy,"Dr. Natale said. He outlinedthree questions to be addressed in phaseII studies: whether ZD6474 has singleagentactivity; whether activity is due toinhibiting EGFR tyrosine kinase, VEGFRtyrosine kinase, or both; and whether it can be combined with chemotherapy.
This study enrolled 168 patients withlocally advanced or metastatic(stage IIIB/IV) NSCLC, afterfailure of at least one platinum-based chemotherapybecause of toxicity or tumorprogression. Brain metastaseswere permitted, and squamouscell histology was not excluded.Patients were randomizedto daily oral doses of ZD6474 (300 mg,n = 83) or gefitinib (250 mg, n = 85)until disease progression or evidence oftoxicity (Part A). After a 4-week washoutperiod, eligible patients then had the optionof crossing over to the alternativetreatment, which continued until diseaseprogression or toxicity. The primary endpointsin Part A were progression-freesurvival (PFS) and safety/tolerability.
Median PFS in Part A (before crossover)was 11.9 weeks for ZD6474 and 8.1weeks for gefitinib (HR 0.69, P = .025).The objective response rate was 8% withZD6474 vs 1% with gefitinib. Diseasecontrol lasted more than 8 weeksin 45% of patients receivingZD6474 and in 34% of patientsreceiving gefitinib. Adverseevents with ZD6474 includeddiarrhea (grade 3-4, 8.4%),rash (grade 3-4, 4.8%) andasymptomatic QTc prolongation(all grade 1, 20.5%).In Part B of the study, 29 patientscrossed over from ZD6474 to gefinitiband 32 crossed over from gefitinib toZD6474. Dr. Natale said that disease controllasting for more than 8 weeks wasachieved in 24% of patients who switchedfrom ZD6474 to gefitinib and in 43% ofthose who switched from gefitinib toZD6474.
Overall survival was 6.1 months in patientsinitially randomized to ZD6474 and7.4 months in patients initially randomized to gefitinib (P = NS). Dr. Natale saidthat the investigators have no clear explanationfor why improvement in PFSdid not translate into an overall survivaladvantage, but they suspect that the crossoverdesign confounded the survivalanalysis.
He concluded that since this studyachieved its primary efficacy objective ofprolonging PFS, compared with gefitinib,the data demonstrate that ZD6474 andgefitinib are both active in NSCLC andsupport further confirmatory trials ofZD6474 monotherapy.
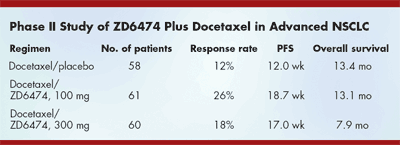
M.D. Anderson Cancer Center investigatorJohn V. Heymach, MD, and his colleagues reported follow-up resultsfrom a phase II trial of ZD6474 plusdocetaxel in patients with previouslytreated stage III/IV NSCLC (abstract7016). The study was designed with an80% power to detect a 50% prolongationin PFS at P < .20. The investigatorsjustified setting the significant level atP < .20 rather than the more usual .05because the trial was "designed to assessthe potential for further investigation."
Dr. Heymach reported an increase inPFS from 12.0 weeks with placebo/docetaxel to 18.7 weeks with 100 mg/dof ZD6474 plus docetaxel and 17.0 weekswith 300 mg/d ZD6474 plus docetaxel.There were no differences in overall survivalamong the three treatment arms (see Table on page 18). He concluded,"The study met its primary endpoint byprolonging progression-free survival by57% [6.7 weeks on the 100 mg/d ZD6474arm]." He offered several possible reasons for the discordance between the progression-free and overall survival results,including patient gender and subsequenttherapy after the study.
Randomized Study
Based on this study, Dr. Heymach saidthat a randomized phase III study ofZD6474 100 mg plus docetaxel vs docetaxelalone in stage III/IV NSCLC afterfailure of first-line therapy will enroll1,240 patients, with a primary objectiveof producing a 25% prolongation in progression-free survival.