26 Prediction of Chemotherapy Benefit by MammaPrint® in Patients With HR+HER2- Early-Stage Breast Cancer From Real-World Evidence Studies
Background
The 70-gene signature test MammaPrint (MP) determines distant recurrence (DR) risk in early-stage breast cancer. In the phase 3 MINDACT trial (NCT00433589), MP identified patients with low-risk disease who have excellent outcomes without chemotherapy (CT). There remains a clinical need to identify biomarkers that predict CT benefit among patients with hormone receptor-positive (HR+)/HER2-negative (HER2–) early-stage breast cancer. Here we examined MP in predicting pathological complete response (pCR) to neoadjuvant chemotherapy (NCT), and 5-year CT benefit among patients who received CT or endocrine therapy (ET) alone.
Methods
Of 901 patients with HR+/HER2– early-stage breast cancer included, 426 enrolled in NBRST (NCT01479101) received neoadjuvant CT. The remaining 475 patients were enrolled in the prospective FLEX trial (NCT03053193) with a 5-year follow-up: 181 received CT (with or without ET), and 294 received ET only. Logistic regression was used to estimate the likelihood of pCR and 5-year DR risk (including breast cancer-specific death) for CT vs ET, as a continuous function of the MP index, defined as ultralow (UL; 1.000-0.356), low risk (LR; 0.355-0.001), high 1 (H1; 0.000 to –0.569), and high 2 (H2; –0.570 to –1.000).
Results
Range (%) of pCR to NCT and 5-Year DR Risk in CT and ET Groups
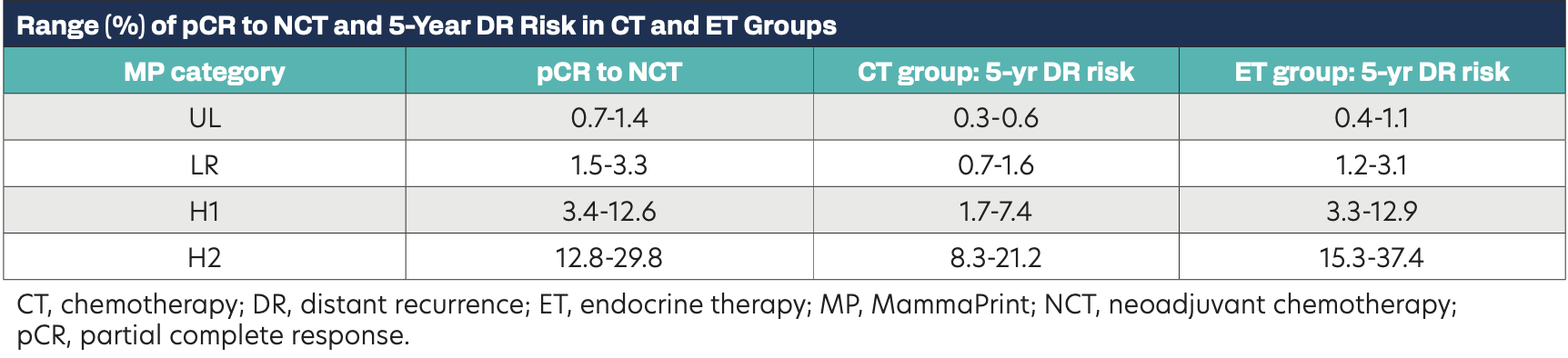
Patients treated with neoadjuvant CT were significantly more likely to achieve pCR as MP risk increased, with up to 30% pCR observed in H2 tumors (P < .001). The MP index demonstrated effective performance for predicting 5-year DR risk in the ET group, with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.73 (P = .005), and the CT group, with an AUC of 0.77 (P = .008). Patients treated with ET only had greater 5-year DR risk with increasing MP risk compared with those with CT. Comparing ET vs CT groups, a less than 1.5% and a less than 1.0% difference in 5-year DR risk was observed for MP indices in the LR and UL range, respectively. In contrast, CT benefit increased with increasing MP risk, with a 2% to 7% absolute risk difference observed in H1, and an 8% to 16% difference among H2 tumors.
Conclusions
These data show that MP indices within LR and UL range exhibit low chemosensitivity and do not derive significant CT benefit, consistent with results in MINDACT. Conversely, the increasing CT benefit observed with increasing MP risk is consistent with the 50% relative reduction in recurrence risk reported by Knauer et al. Overall these findings indicate the utility of MammaPrint to predict neoadjuvant and adjuvant CT benefit in patients with HR+/HER2– early-stage breast cancer.
