3 Things You Should Know About Adverse Events With Targeted Therapies for DLBCL
Tips from experts on how to think about and manage adverse events in patients with diffuse large B-cell lymphoma.
The experts

RELEASE DATE: February 1, 2025
EXPIRATION DATE: February 1, 2026
LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
- Recognize adverse events associated with the different classes of targeted therapies used in relapsed/refractory DLBCL
- Formulate strategies for the monitoring, identification, and mitigation of toxicities linked to targeted agents in patients with DLBCL
Accreditation/Credit Designation
Physicians’ Education Resource, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credit.™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgment of commercial support
This activity is supported by an educational grant from Pfizer Inc.
Off-label disclosure/disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a healthcare professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members and do not reflect those of PER or any company that provided commercial support for this activity.
Instructions for participation/how to receive credit
- Read this activity in its entirety.
- Go to https://www.gotoper.com/bcme24taewttdlbcl-postref to access and complete the posttest.
- Answer the evaluation questions.
- Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.
Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) are adverse events (AEs) of particular concern with bispecific antibodies (BsAbs) and chimeric antigen receptor (CAR) T-cell therapies used in treating diffuse large B-cell lymphoma (DLBCL).1 Here are 3 things you should know about managing these AEs.
1. CRS and ICANS are AEs of particular concern with bispecific antibody and CAR T-cell therapy.
The hallmark symptom associated with CRS is fever. Hypoxia, hypotension, tachypnea, nausea, headache, fatigue, myalgia, and malaise can also occur, typically after administration of the CAR T-cell product or the first full dose of bispecific antibodies. ICANS can cause delirium, dysgraphia, tremor, lethargy, difficulty concentrating, agitation, confusion, expressive aphasia, apraxia, depressed level of consciousness, encephalopathy, and seizures. CRS and ICANS can result from administration of either CAR T-cell therapy or bispecific antibodies (Table 1).2-5
Table 1
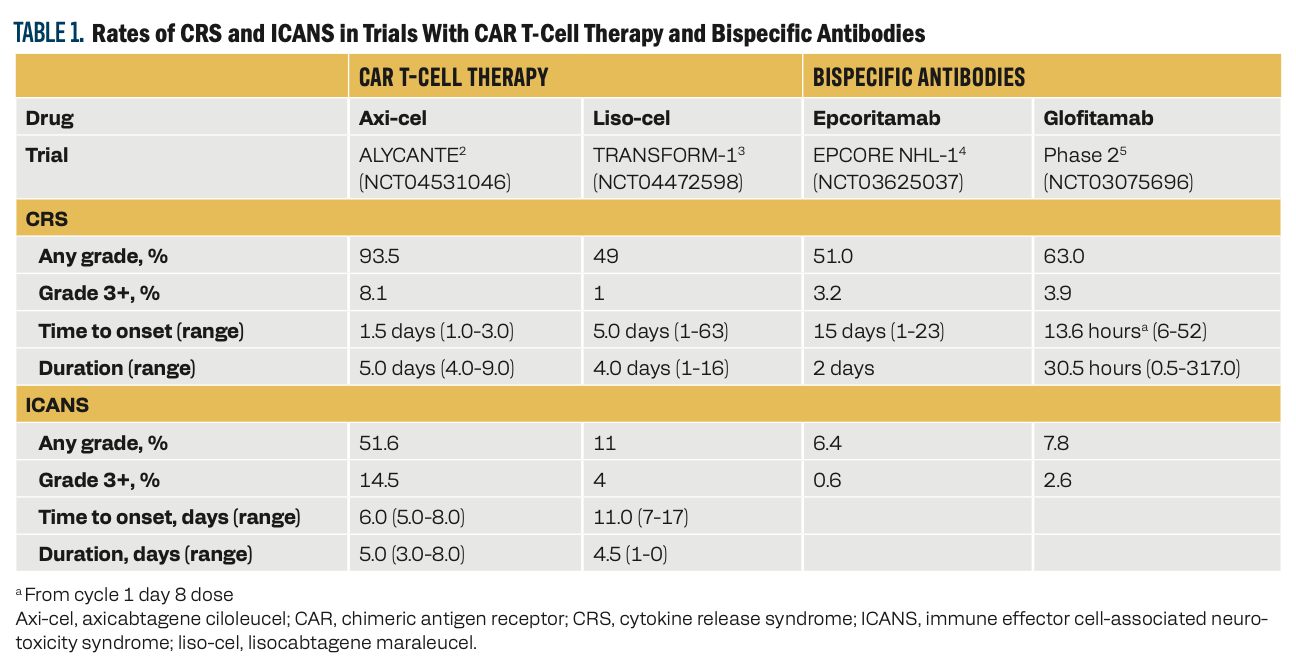
2. Guidelines help grade and manage CRS and ICANS.
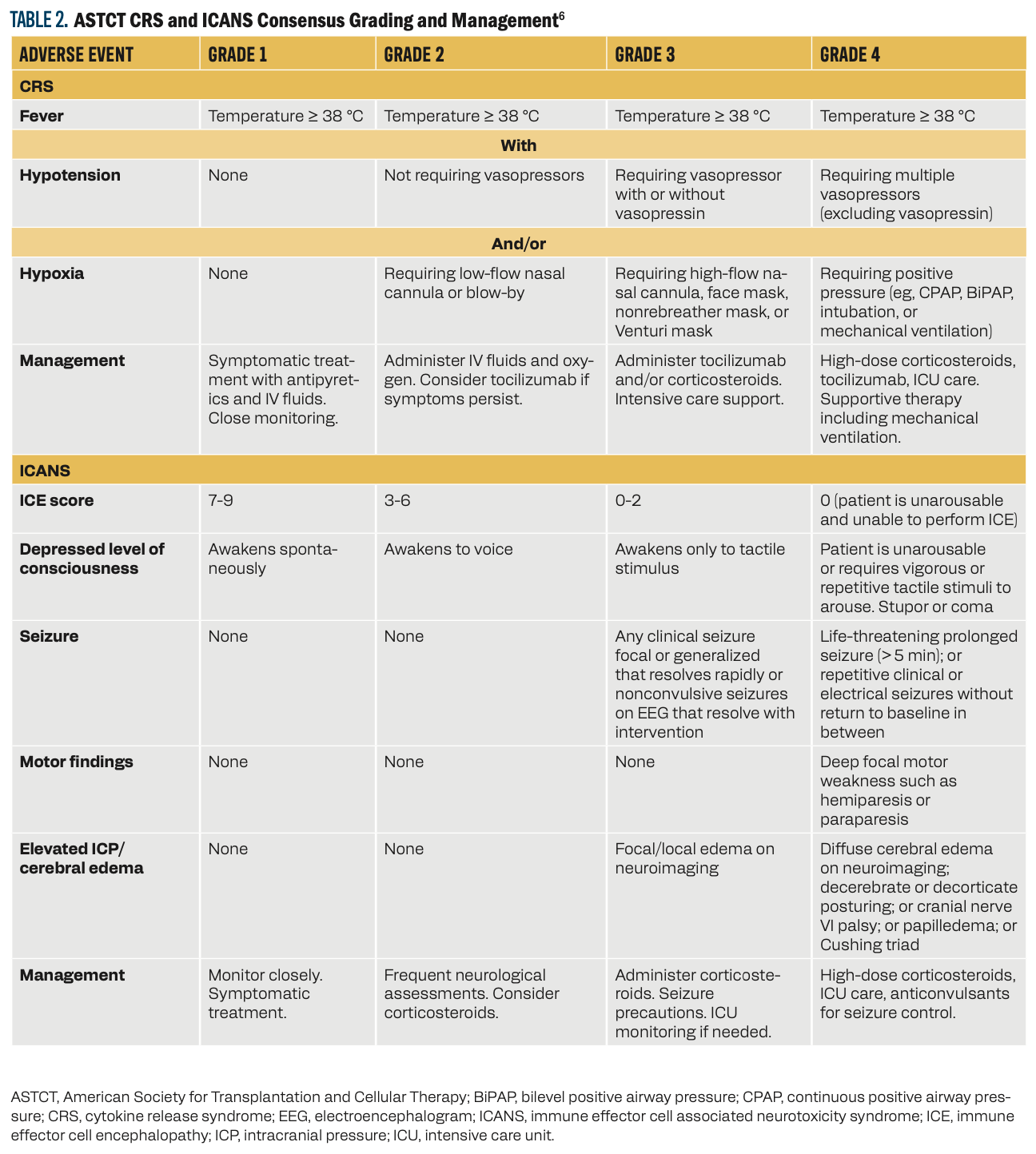
Several grading systems have been developed for CRS and ICANS. The American Society for Transplantation and Cellular Therapy (ASTCT) created a consensus tool to harmonize the various definitions and grading systems for both AEs (Table 2).6
Table 2
3. Antibody-drug conjugates are associated with unique toxicity profiles.
Guidelines for the treatment of DLBCL include several antibody-drug conjugates (ADCs) in various lines of therapy.7 Each ADC presents a unique toxicity profile. Polatuzumab vedotin (pola) can be coadministered with bendamustine with or without rituximab, a combination that can pose a challenge to patients with preexisting neuropathy. In a single-arm phase 1b/2 trial (NCT02257567), the most common AEs of grade 3 or greater in the pola plus bendamustine and rituximab (pola-BR) and BR only arms were neutropenia (46.2% and 33.3%), anemia (28.2% and 17.9%), thrombocytopenia (41% and 23.1%), and infection (23.1% and 20.5%), respectively.8 Peripheral neuropathy is an AE of particular concern with pola and was reported in 43.6% of patients. All cases were grade 1 or 2 and were resolved in most patients. Growth factor support for neutropenia and dose reductions or delays are management techniques for AEs due to pola.
Loncastuximab tesirine (lonca) is recommended as a third-line regimen for patients with DLBCL.7 In the multicenter, single-arm, phase 2 LOTIS-2 trial (NCT03589469) of 145 patients, the most common grade 3 or higher treatment-emergent AEs (TEAEs) were neutropenia (26%), thrombocytopenia (18%), and increased gamma-glutamyl transferase (17%). A total of 39% of patients experienced serious AEs. Eight fatal TEAEs were reported, but none were attributed to lonca.9 Management of AEs associated with lonca includes dose delays or reductions and growth factor support. To mitigate these AEs, treatment with lonca is given at a higher dose for the first 2 doses and then the dose is lowered. Prophylaxis with dexamethasone is also used, and patients are advised to avoid sun exposure.
Brentuximab vedotin (BV) has not yet received FDA approval for use in patients with DLBCL; however, the phase 3 ECHELON-3 trial (NCT04404283) demonstrated improved outcomes with BV added to a regimen of lenalidomide and rituximab (BV-R2) vs lenalidomide and rituximab alone (R2).10 In this trial, in the BV-R2 and R2 arms, grade 3 TEAEs were reported in 88% and 77% of participants, serious TEAEs were reported in 60% and 50% of participants, and grade 5 TEAEs were reported in 12% and 8% of participants, respectively. The most common TEAEs were neutropenia (46% vs 32%), anemia (29% vs 27%), and diarrhea (31% and 23%), respectively. Peripheral neuropathy of any grade was reported in 31% of patients in the BV-R2 arm and 24% of patients in the R2 arm. Grade 3 peripheral neuropathy occurred in 6% and 2% of patients, respectively. AEs were typically managed with dose delays or reductions and growth factor support.
Key References
4. Thieblemont C, Karimi YH, Ghesquieres H, et al. Epcoritamab in relapsed/refractory large B-cell lymphoma: 2-year follow-up from the pivotal EPCORE NHL-1 trial. Leukemia. 2024;38(12):2653-2662. doi:10.1038/s41375-024-02410-8
6. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells.Biol Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j.bbmt.2018.12.758
10. Kim JA, Hahn U, Kim W-S, et al. Brentuximab vedotin in combination with lenalidomide and rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma: results from the phase 3 ECHELON-3 study. J Clin Oncol. 2024;42(suppl 17):LBA7005. doi:10.1200/JCO.2024.42.17_suppl.LBA7005
For full reference list, visit
https://www.gotoper.com/bcme24taewttdlbcl-postref
CME Posttest Questions
Claim Your CME Credit at https://www.gotoper.com/bcme24taewttdlbcl-postref
1. In the phase 2 EPCORE NHL-1 study (NCT03625037) that assessed the efficacy and safety of epcoritamab, a CD3 × CD20 T-cell–engaging bispecific antibody, 51% of patients developed CRS. What was the most common time to develop it?
A. After 1 cycle
B. Following the first full dose
C. After receiving 2 cycles
D. After receiving 3 cycles
2. In the phase 3 ECHELON-3 study (NCT04404283), which of the
following was the most frequently occurring adverse event observed in patients who received brentuximab-vedotin in combination with lenalidomide-rituximab?
A. Anemia
B. Nausea
C. Neutropenia
D. Peripheral neuropathy
3. You are treating a patient with relapsed DLBCL after a recent
CD19-directed CAR T-cell transplant. The patient developed myalgias, fever, tachycardia, and hypotension without hypoxia after the first dose. Upon further evaluation and workup, the patient is diagnosed with
grade 2 CRS. Which of the following treatment approaches would be most appropriate?
A. Monitor the patient carefully, but no other intervention needed.
B. Monitor carefully as outpatient for 8 hours, initiate IV fluids, and provide acetaminophen.
C. Admit to the hospital and provide IV fluids, acetaminophen, and corticosteroids.
D. Admit to the hospital, provide corticosteroids and tocilizumab.
To learn more about this topic, including information on the role of biomarkers in clinical decision-making and treatment strategies for diffuse large B-cell lymphoma (DLBCL), go to https://www.gotoper.com/bcme24taewttdlbcl-activity
CME Provider Contact information
Physicians’ Education Resource®, LLC
2 Commerce Drive, Suite 110, Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com

Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.