3 Things You Should Know About Future Directions for BsAbs and ADCs in NSCLC
The latest advancements in bispecific antibodies and antibody-drug conjugates continue to improve the personalized treatment of advanced non-small cell lung cancer.
The experts.

LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
• Integrate the latest evidence and disease- and patient-specific characteristics into personalized treatment plans for advanced NSCLC
• Describe the unique mechanisms of emerging bispecific antibodies and ADCs being investigated for the treatment of advanced NSCLC
• Assess the implications of recent clinical trial evidence on the potential roles for emerging bispecific antibodies and ADCs in advanced NSCLC
Acknowledgement of Commercial Support
This activity is supported by an educational grant from Summit Therapeutics, Inc.
Off-Label Disclosure and Disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical
condition. The opinions expressed in the content are solely those of the individual faculty members, and do not reflect those of PER® or any company that provided commercial support for this activity.
Introduction
Bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs) are rapidly evolving classes of therapeutics that have made an impact on the treatment of advanced non–small cell lung cancer (NSCLC). Here are 3 things you should know about future directions for BsAbs and ADCs in NSCLC.
1/ BsAbs and ADCs target tumor cells for destruction.
BsAbs bind 2 different antigens or epitopes simultaneously.1 In the treatment of patients with advanced NSCLC, these antigens could be CD3 on T cells and a tumor neoantigen on cancer cells, designating the BsAb as a T-cell engager. Alternatively, non–T-cell engager BsAbs bind 2 different tumor neoantigens and can kill the cancer cell via multiple strategies, including blocking ligand binding to the receptor, initiating receptor-antibody degradation, or triggering macrophage-mediated trogocytosis and antibody-dependent cellular cytotoxicity (Figure 1).2,3 The dual targeting of BsAbs can yield higher specificity and lower toxicity than single-target antibodies. Targeting multiple pathways may avoid resistance and provide the opportunity to bind to tumor cells that do not express one or the other receptor. ADCs, by contrast, bind to a single target on the tumor cell and are internalized via endocytosis to release a cytotoxic payload to the target cell, and possibly neighboring cells, through the bystander effect.3 In the NSCLC field, some ADCs—including telisotuzumab vedotin (Teliso-V), patritumab deruxtecan, and trastuzumab deruxtecan (T-DXd)—are biomarker-selected. TROP2-targeting antibodies, including datopotamab deruxtecan (Dato-DXd), sacituzumab govitecan, and sacituzumab tirumotecan, are considered biomarker-agnostic, although this remains an active area of investigation.
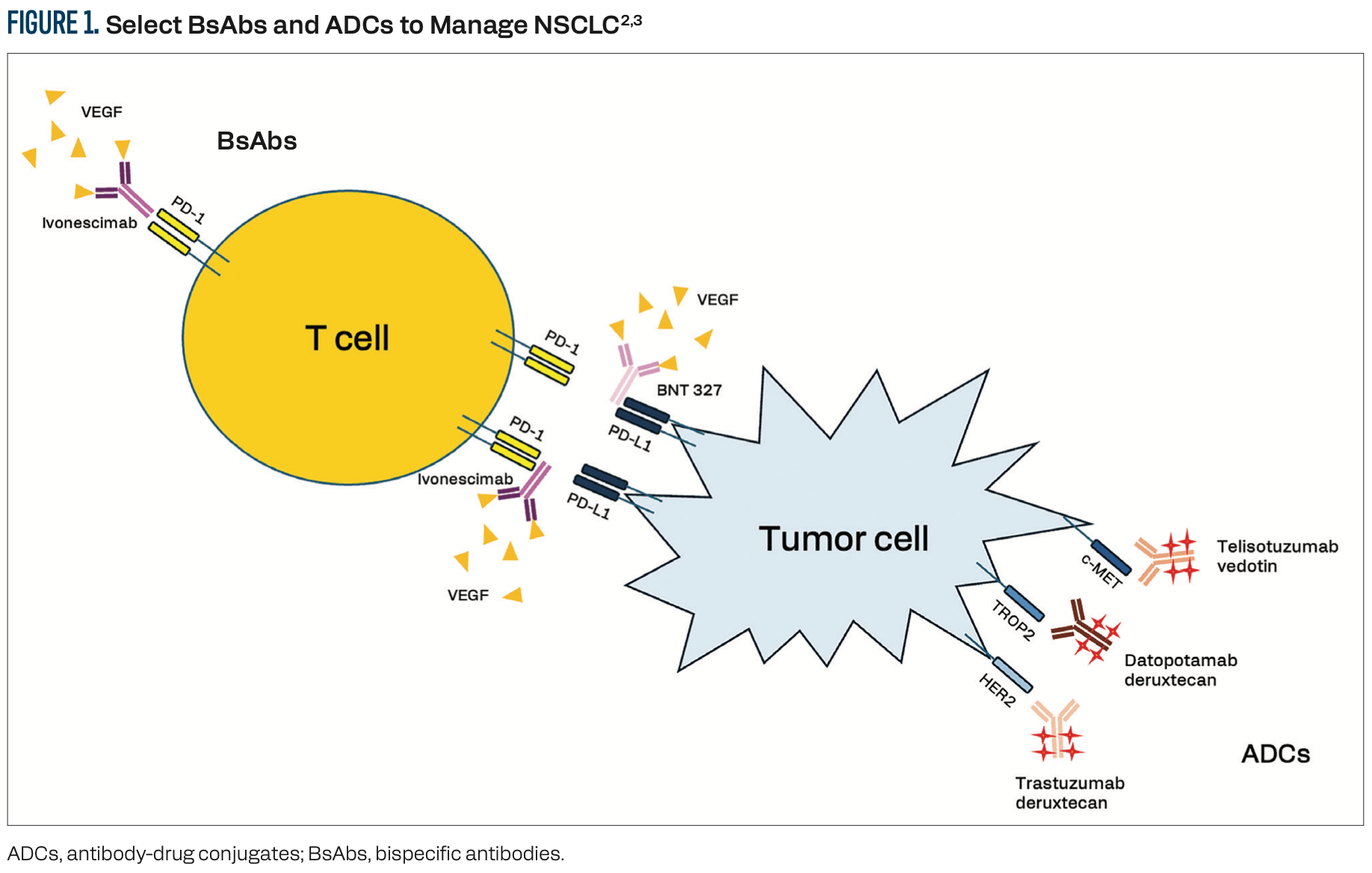
2/ BsAbs targeting VEGF may create more favorable tumor microenvironments.
FDA-approved BsAbs for patients with NSCLC include amivantamab, which binds MET and EGFR, and zenocutuzumab, which blocks HER2-HER3 dimerization and NRG1 fusion protein interactions with HER3.4, 5 Ivonescimab (AK112) is a first-in-class VEGF x PD-1 BsAb that engages in cooperative binding such that the binding of either antigen enhances binding avidity to the other.6 VEGF is a potent drug target due to its role in angiogenesis and immune suppression.7 Inhibiting VEGF inhibits tumor vascularization and has the potential to reprogram the tumor microenvironment to enhance infiltration by immune cells. Inhibiting the PD-1/PD-L1 signaling pathway may further enhance the immune-permissive tumor microenvironment. In a phase 2 multicenter trial (NCT04736823), 4 cycles of ivonescimab plus pemetrexed and carboplatin chemotherapy, followed by ivonescimab maintenance therapy, produced objective response rates (ORR) of up to 68.4% in patients with EGFR-mutated advanced NSCLC who had progressed after prior EGFR tyrosine kinase inhibitor (TKI) therapy.8
The HARMONi family of clinical trials is investigating ivonescimab in a variety of settings, including the first line, second line, in combination with chemotherapy, and as monotherapy. In the phase 3 HARMONi-A trial (NCT05184712), 322 patients with advanced or metastatic NSCLC harboring an EGFR mutation and who experienced disease progression on frontline EGFR TKI therapy were randomly assigned 1:1 to receive ivonescimab with chemotherapy or placebo with chemotherapy across 55 sites in China.9 The trial met its primary end point with statistically significantly higher progression-free survival (PFS) with ivonescimab vs placebo (Table).9 PFS benefit was seen regardless of the type of EGFR mutation and the presence of brain metastases.

The phase 3 HARMONi trial (NCT06396065) was the same study design conducted in 438 patients globally.10 Again, ivonescimab demonstrated a statistically significant higher PFS than the placebo (Table).10 At the final overall survival (OS) analysis, the median OS in the ivonescimab arm was 16.8 months (95% CI, 14.3-19.0) vs 14.0 months (95% CI, 12.8-15.7) in the placebo arm (HR, 0.79; 95% CI, 0.62-1.01; P = .0570).
Ivonescimab monotherapy was compared with pembrolizumab monotherapy as first-line treatment in 398 patients with PD-1–positive advanced NSCLC across 55 sites in China in the phase 3 HARMONi-2 trial (NCT05499390).11 The median PFS with ivonescimab was again significantly higher than that with pembrolizumab (Table).11
Additional VEGF x PD-(L)1 BsAbs are under investigation in clinical trials. BNT 327 is a VEGF x PD-L1 BsAb that demonstrated an overall ORR of 60.9% (95% CI, 47.9%-72.9%) and a disease control rate of 95.3% (95% CI, 86.9%-99.0%) in combination with chemotherapy in patients with EGFR-mutated NSCLC who had experienced progression after EGFR TKI treatment in a single-arm, phase 2 study (NCT05756972).12 In patients whose tumors were PD-L1 negative, the ORR was 46.4% (95% CI, 27.5%-66.1%) vs 92.3% (95% CI, 64.0%-99.8%) in patients whose tumors had high expression of PD-L1.
3/ ADCs targeting a variety of tumor antigens are gaining approval to manage NSCLC.
Several ADCs have been approved to treat patients with NSCLC as second-line therapies after chemo-immunotherapy or TKI. T-DXd was granted tumor-agnostic approval for HER2-positive solid tumors based on the DESTINY family of clinical trials.13 Dato-DXd was recently approved to treat EGFR-mutated NSCLC based on pooled results from the phase 2 TROPION-Lung05 (NCT04484142) and phase 3 TROPION-Lung01 (NCT04656652) trials.14 In the 117 patients with EGFR-mutated NSCLC who made up the pooled analysis, the confirmed ORR was 43% (95% CI, 34%-52%), including 5 complete responses.15 The median duration of response was 7.0 months (95% CI, 4.2-9.8). The median PFS was 5.8 months (95% CI, 5.4-8.2), and the median OS was 15.6 months (95% CI, 13.1-19.0).Grade 3 or higher treatment-related AEs were reported in 23% of patients.
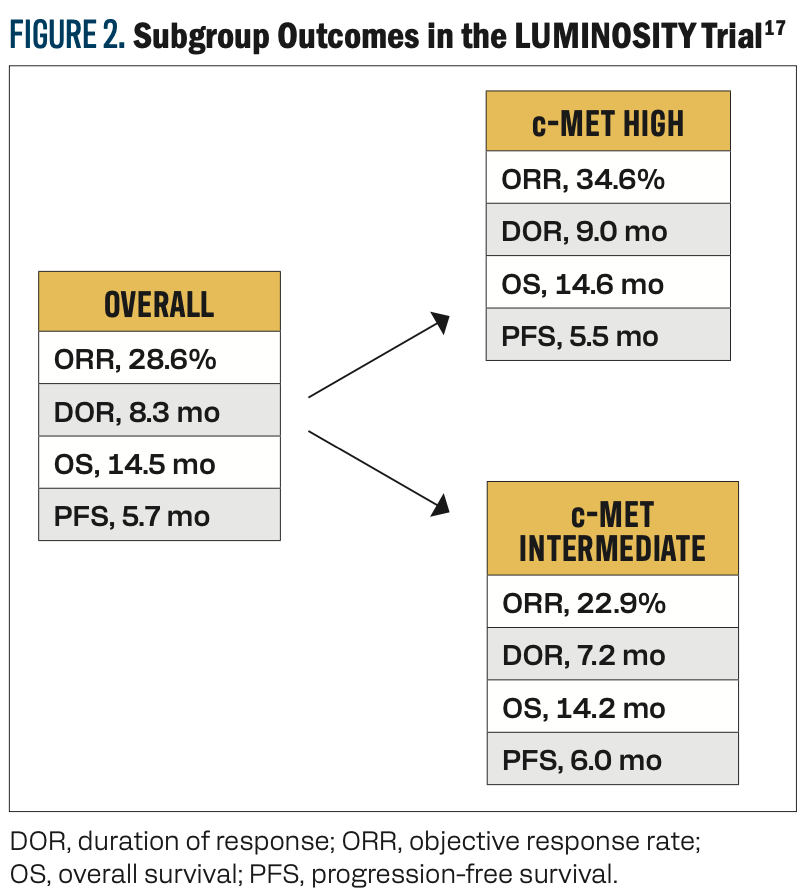
Teliso-V was recently granted accelerated approval to treat patients with advanced or metastatic nonsquamous NSCLC with high c-Met overexpression based on results from the phase 2 LUMINOSITY trial (NCT03539536).16 In this study, 172 patients with nonsquamous EGFR wild-type NSCLC who had experienced disease progression on at least 2 previous lines of therapy were given Teliso-V every 2 weeks.17 Patients with high c-Met expression (3+ immunohistochemistry [IHC] staining in at least 50% of cells) generally experienced better outcomes than patients with intermediate c-Met expression (3+ IHC staining in 25%-50% of cells; Figure 2).17 The most common AE of grade 3 or higher was peripheral sensory neuropathy (7%).
Approval of additional ADCs and BsAbs and expanded indications for currently approved agents may reshape the treatment landscape for patients with NSCLC in the near future.
References
1. Goebeler ME, Stuhler G, Bargou R. Bispecific and multispecific antibodies in oncology: opportunities and challenges. Nat Rev Clin Oncol. 2024;21(7):539-560. doi:10.1038/s41571-024-00905-y
2. Guidi L, Etessami J, Valenza C, et al. Bispecific antibodies in hematologic and solid tumors: current landscape and therapeutic advances. Am Soc Clin Oncol Educ Book. 2025;45(3):e473148. doi:10.1200/edbk-25-473148
3. Zanchetta C, De Marchi L, Macerelli M, et al. Antibody-drug conjugates in non-small cell lung cancer: state of the art and future perspectives. Int J Mol Sci. 2024;26(1):221. doi:10.3390/ijms26010221
4. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion–mutated non–small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391-3402. doi:10.1200/jco.21.00662
5. Schram AM, Goto K, Kim D-W, et al; eNRGy Investigators. Efficacy of zenocutuzumab in NRG1 fusion-positive cancer. N Engl J Med. 2025;392(6):566-576. doi:10.1056/NEJMoa2405008
6. Zhong T, Zhang L, Huang Z, et al. Design of a fragment crystallizable-engineered tetravalent bispecific antibody targeting programmed cell death-1 and vascular endothelial growth factor with cooperative biological effects. iScience. 2025;28(3):111722. doi:10.1016/j.isci.2024.111722
7. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(pt 2):117-124. doi:10.1016/j.semcancer.2017.12.002
8. Zhao Y, Chen G, Chen J, et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. EClinicalMedicine. 2023;62:102106. doi:10.1016/j.eclinm.2023.102106
9. HARMONi-A Study Investigators; Fang W, Zhao Y, Luo Y, et al. Ivonescimab plus chemotherapy in non-small cell lung cancer with EGFR variant: a randomized clinical trial. JAMA. 2024;332(7):561-570. doi:10.1001/jama.2024.10613
10. Goldman J, Passaro A, Laskin J, et al. Ivonescimab vs placebo plus chemo, phase 3 in patients with EGFR+ NSCLC progressed with 3rd gen EGFR-TKI treatment: HARMONi. Presented at: 2025 World Conference on Lung Cancer; September 6-9, 2025; Barcelona, Spain.
11. Xiong A, Wang L, Chen J, et al. Ivonescimab versus pembrolizumab for PD-L1-positive non-small cell lung cancer (HARMONi-2): a randomised, double-blind, phase 3 study in China. Lancet. 2025;405(10481):839-849. doi:10.1016/s0140-6736(24)02722-3
12. Wu YL, Wang Z, Cheng Y, et al. A phase II safety and efficacy study of PM8002/BNT327 in combination with chemotherapy in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Ann Oncol. 2024;35(suppl 2):S804. doi:10.1016/j.annonc.2024.08.1312
13. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors. FDA. April 5, 2024. Accessed October 6, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2
14. FDA grants accelerated approval to datopotamab druxtecan-dlnk for EGFR-mutated non-small cell lung cancer. FDA. June 23, 2025. Accessed October 6, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-datopotamab-deruxtecan-dlnk-egfr-mutated-non-small-cell-lung-cancer
15. Ahn MJ, Lisberg A, Goto Y, et al. A pooled analysis of datopotamab deruxtecan in patients with EGFR-mutated NSCLC. J Thorac Oncol. Published online June 12, 2025. doi:10.1016/j.jtho.2025.06.002
16. FDA grants accelerated approval to telisotuzumab vedotin-tllv for NSCLC with high c-Met protein overexpression. FDA. May 14, 2025. Accessed October 7, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-telisotuzumab-vedotin-tllv-nsclc-high-c-met-protein-overexpression
17. Camidge DR, Bar J, Horinouchi H, et al. Telisotuzumab vedotin monotherapy in patients with previously treated c-Met protein-overexpressing advanced nonsquamous EGFR-wildtype non-small cell lung cancer in the phase II LUMINOSITY trial. J Clin Oncol. 2024;42(25):3000-3011. doi:10.1200/jco.24.00720
To learn more about this topic, including information on additional bispecific antibodies and antibody-drug conjugates under investigation, go to https://www.gotoper.com/nsclc25adcs-activity
CME Provider Contact information
Physicians’ Education Resource®, LLC
259 Prospect Plains Road,
Bldg H,
Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com
