3 Things You Should Know About Hemolytic Anemias
Hemolytic anemias are a collection of rare but severe diseases including paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
Hemolytic anemias are a collection of rare but severe diseases including paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS), which occur in less than 1 person per 100,000, and warm autoimmune hemolytic anemia (wAIHA), which occurs in up to 3 people per 100,000.1-3 Here are 3 things you should know about hemolytic anemias.
1 Anti-C5 targeted therapy is a mainstay of PNH and aHUS treatment
In both PNH and aHUS, overactivity in the alternative complement cascade can lead to anemia, kidney damage, and other systemic dysregulation.4,5 Several drugs have been developed targeting proteins of the alternative complement cascade to treat symptoms of PNH and aHUS (Figure). The C5 inhibitors eculizumab and ravulizumab were compared head-to-head in the 301 and 302 trials (NCT02946463 and NCT03056040, respectively).6,7 Ravulizumab demonstrated noninferiority to standard-of-care eculizumab in terms of transfusion avoidance, least-square mean (LSM) percent change in lactate dehydrogenase (LDH) levels, change in FACIT-Fatigue score, percentage of patients experiencing breakthrough hemolysis, and hemoglobin (Hgb) stabilization.
Figure. Alternative Complement Cascade and Inhibitors
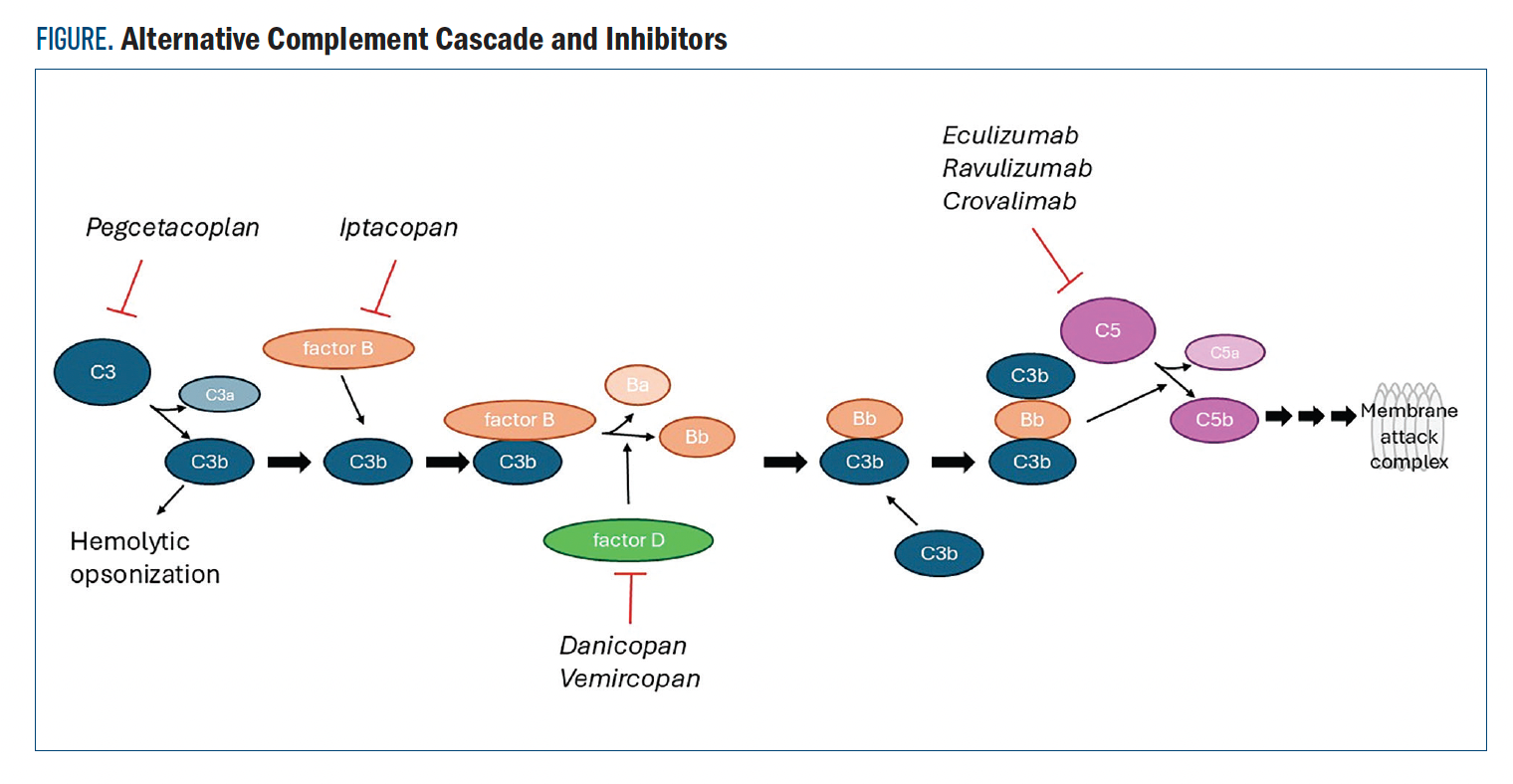
Crovalimab is the most recently approved therapy for PNH based on results from the phase 3, single-arm COMMODORE 3 trial (NCT04654468) in 51 complement inhibitor-naive patients with PNH who received at least 4 transfusions of packed red blood cells in the 12 months prior to screening.8 The estimated mean proportion of patients with hemolysis control from week 5 through week 25 was 78.7% (95% CI, 67.8%-86.6%). The proportion of patients with transfusion avoidance from baseline through week 25 was 51.0% vs 0% within 24 weeks of prescreening (P < .0001). No treatment discontinuations were due to adverse events (AEs).
Investigations into novel C5-targeting agents include a phase 2 trial (NCT04811716) of the combination of an anti-C5 monoclonal antibody, pozelimab, and cemdisiran, a small interfering RNA that suppresses C5 production in the liver.9 During the 28-week open-label treatment period, 83.3% of the 24 trial participants maintained control of LDH (LDH levels were no greater than 1.5 times the upper limit of normal [ULN]) at all time points. Additionally, 75% of participants were considered to have stabilized Hgb, (no blood transfusions required and Hgb levels of at least 2 g/dL were maintained). Breakthrough hemolysis requiring a blood transfusion occurred in 2 patients. No severe treatment-emergent AE (TEAEs) were considered to be treatment-related.
2 Additional PNH and aHUS treatments target upstream components of the alternative complement cascade
Pegcetacoplan is a C3 inhibitor (Figure) approved for use in PNH. In the phase 3 PRINCE trial (NCT04085601) in complement inhibitor-naive patients with PNH, pegcetacoplan demonstrated improved Hgb stabilization from baseline to week 26 in 85.7% of 35 patients vs 0% of 18 control patients receiving supportive care (difference, 73.1%; 95% CI, 57.2%-89.0%; P < .0001).10 Pegcetacoplan also improved change from baseline LDH with an LSM change of –1870.5 U/L vs –400.1 U/L in the control arm (difference, –1470.4 U/L; 95% CI, –2113.4 to –827.3 U/L; P <.0001). Treatment with pegcetacoplan did not contribute to any serious AEs.
Iptacopan is a first-in-class oral factor B inhibitor (Figure) that demonstrated improved Hgb levels in patients with PNH in a pair of phase 3 trials.11 In the APPLY-PNH study (NCT04558918), patients who had received prior eculizumab or ravulizumab were randomly assigned to continue with anti-C5 therapy or switch to iptacopan. The APPOINT-PNH study (NCT04820530) evaluated patients who had not received complement-inhibitor therapy and had LDH levels that were at least 1.5 times the ULN. In the 2 trials, 85% and 94% of patients receiving iptacopan experienced an increase in Hgb levels of at least 2 g/dL from baseline, respectively, vs 0% of patients in the APPLY-PNH study who continued with anti-C5 therapy. The most common AE reported among patients receiving iptacopan in these trials was headache.
Danicopan is a selective factor D inhibitor (Figure) evaluated as an add-on therapy to ravulizumab or eculizumab in the phase 3 ALPHA trial (NCT04469465).12 A total of 73 patients with PNH who had been on ravulizumab or eculizumab for at least 6 months were randomly assigned (2:1) to add danicopan or placebo to their regimen. At 12 weeks, add-on danicopan increased Hgb from baseline by a LSM difference of 2.94 g/dL (95% CI, 2.52-3.36 g/dL) vs 0.50 g/dL (95% CI, –0.13 to 1.12 g/dL). No serious AEs were attributed to danicopan.
3 Novel targets are under investigation in wAIHA
Standard of care for patients with wAIHA involves successive use of corticosteroids, rituximab, and splenectomy.13 Fostamatinib is an oral spleen tyrosine kinase (Syk) inhibitor approved for the treatment of chronic immune thrombocytopenia and is under investigation for the treatment of wAIHA in the phase 3 FORWARD trial (NCT03764618).14 A total of 90 patients with wAIHA who had experienced insufficient response to at least 1 prior treatment were randomly assigned (1:1) to receive fostamatinib or placebo. At 24 weeks, after censoring for Hgb values impacted by steroid rescue during screening and excluding 2 patients deemed unlikely to have wAIHA, 33.3% of patients in the fostamatinib arm achieved a durable Hgb response of at least 10 g/dL vs 14.0% of patients in the placebo arm (P = .0395).
Sovleplenib (HMPL-523) is another Syk inhibitor under investigation in patients in China with wAIHA in a phase 2/3 study (NCT05535933).15 A total of 21 patients who had received at least 1 prior therapy were randomly assigned (3:1) to receive sovleplenib or placebo. At 8 weeks, the overall Hgb response rate was 43.8% in the sovleplenib arm vs 0% in the placebo arm.
A retrospective study queried whether the anti-CD38 monoclonal antibody daratumumab could suppress the secretion of wAIHA-inducing autoantibodies from CD38+ plasma cells.16 In 12 patients with steroid and/or rituximab-refractory wAIHA, overall response was 50% with a median duration of response of 5.5 months (range, 2-12 months). Blood samples were prospectively collected from 2 patients and showed complete CD38+ T-cell depletion.
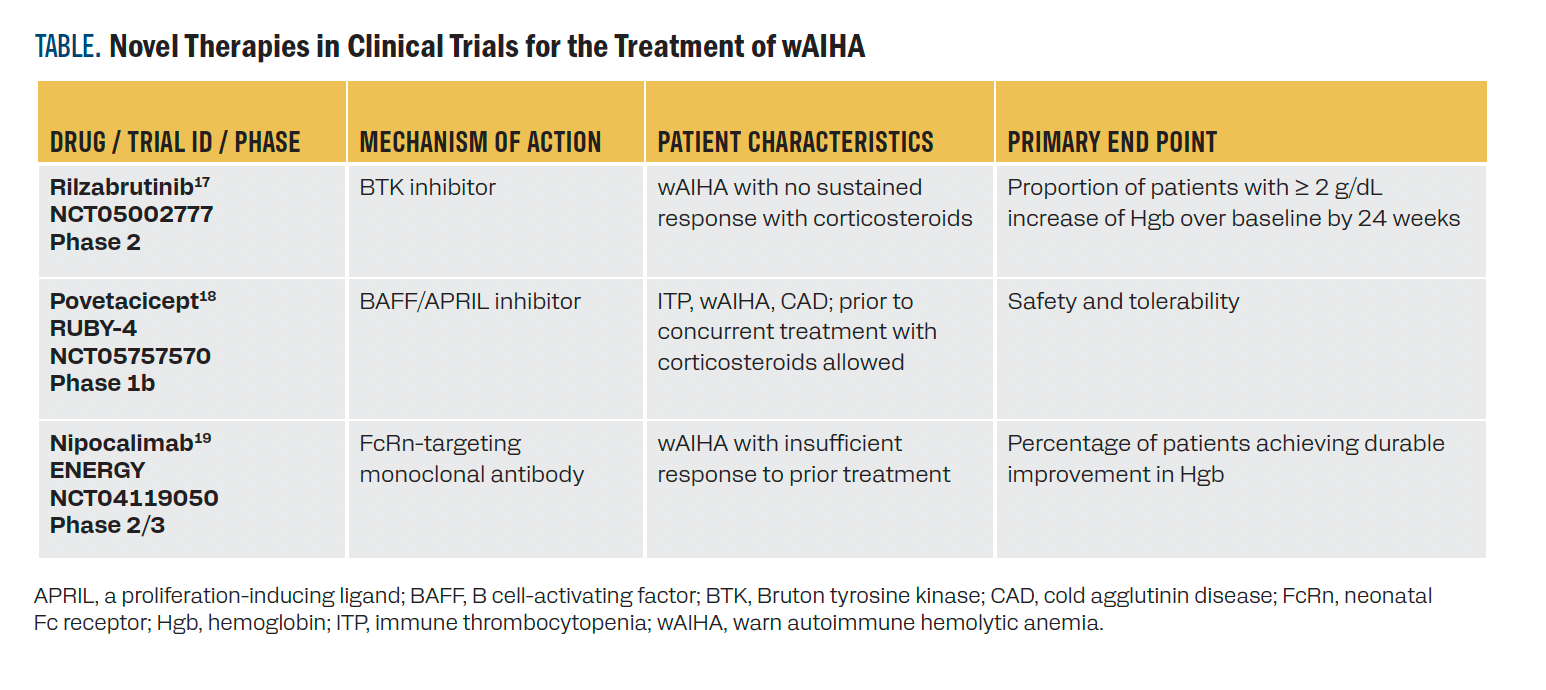
Additional early-phase trials of novel therapies are outlined in the Table.17-19 Rilzabrutinib may inhibit the production of autoimmune antibodies.17 The cytokine antagonist povetacicept suppresses autoimmune antibody production by plasma cells.18 Nipocalimab blocks IgG recirculation, causing a reduction in serum IgG by approximately 90%.19
TABLE. Novel Therapies in Clinical Trials for the Treatment of wAIHA
KEY REFERENCES
8. Liu H, Xia L, Weng J, et al. Efficacy and safety of the C5 inhibitor crovalimab in complement inhibitor-naive patients with PNH (COMMODORE 3): a multicenter, phase 3, single-arm study. Am J Hematol. 2023;98(9):1407-1414. doi:10.1002/ajh.26998
10. Wong RSM, Navarro-Cabrera JR, Comia NS, et al. Pegcetacoplan controls hemolysis in complement inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria. Blood Adv. 2023;7(11):2468-2478. doi:10.1182/bloodadvances.2022009129
14. Kuter DJ, Piatek C, Röth A, et al. Fostamatinib for warm antibody autoimmune hemolytic anemia: phase 3, randomized, double-blind, placebo-controlled, global study (FORWARD). Am J Hematol. 2024;99(1):79-87. doi:10.1002/ajh.27144
19. Murakhovskaya I, Fattizzo B, Cueto D, Perdomo AB, Jouvin M. Efficacy and safety of nipocalimab, an FcRn blocker, in warm autoimmune hemolytic anemia (wAIHA): ENERGY phase 2/3 study design. Hematol Transf Cell Therapy. 2022;44:S11. doi:10.1016/j.htct.2022.09.019
Publisher's Note: The December 1, 2024 CME article entitled "3 Things You Should Know About Hemolytic Anemias" was published with an error. As of the date of publication, rilzabrutinib remains under review for approval in immune thrombocytopenia. This error has been addressed as of January 16, 2025, and content has been adjusted as follows:
The text has been updated to: Rilzabrutinib may inhibit the production of autoimmune antibodies.17
For full list of references visit
https://www.gotoper.com/mxf24ha-activity
RELEASE DATE: December 1, 2024
EXPIRATION DATE: December 1, 2025
LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
• Analyze data for current and emerging therapeutic strategies for PNH, aHUS, and wAIHA
• Determine the implication of recent approvals as well as emerging data for investigational approaches for PNH, aHUS, and wAIHA
Accreditation/Credit Designation
Physicians’ Education Resource, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource, LLC, designates this enduring material for a maximum of .25 AMA PRA Category 1 Credits.™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgement of commercial support
This activity is supported by an educational grant from Janssen Biotech, Inc, administered by Janssen Scientific Affairs, LLC; both are Johnson & Johnson companies.
Off-label disclosure/disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members and do not reflect those of PER or any company that provided commercial support for this activity.
Instructions for participation/how to receive credit
1. Read this activity in its entirety.
2. Go to https://gotoper.com/mxf24ha-postref to access and complete the post test
3. Answer the evaluation questions.
4. Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.

Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.