30 Datopotamab Deruxtecan (Dato-DXd) vs Chemotherapy in Previously-Treated Inoperable or Metastatic Hormone Receptor–Positive, HER2-Negative (HR+/HER2–) Breast Cancer (BC): Primary Results From the Randomised Phase 3 TROPION-Breast01 Trial
Table
Background
The TROP2-directed antibody-drug conjugate datopotamab deruxtecan (Dato-DXd) demonstrated promising activity in patients with heavily pretreated inoperable or metastatic hormone receptor-positive (HR+)/HER2-negative (HER2–) breast cancer in the phase 1 TROPION-PanTumor01 trial (NCT03401385). Here we report primary progression-free survival (PFS) results from the global, phase 3 TROPION-Breast01 trial (NCT05104866).
Materials and Methods
Adult patients with inoperable or metastatic HR+/HER2– breast cancer who had experienced progression on endocrine therapy (ET) and for whom ET was unsuitable, and who had received 1 to 2 prior lines of systemic chemotherapy (CT), were randomly assigned 1:1 to Dato-DXd (6 mg/kg every 3 weeks) or investigator’s choice of CT (investigator choice chemotherapy [ICC]; eribulin, vinorelbine, capecitabine, or gemcitabine) until progression or unacceptable toxicity. Dual primary end points were progression-free survival (PFS) by blinded independent central review (BICR) per
RECIST 1.1, and overall survival (OS).
Results
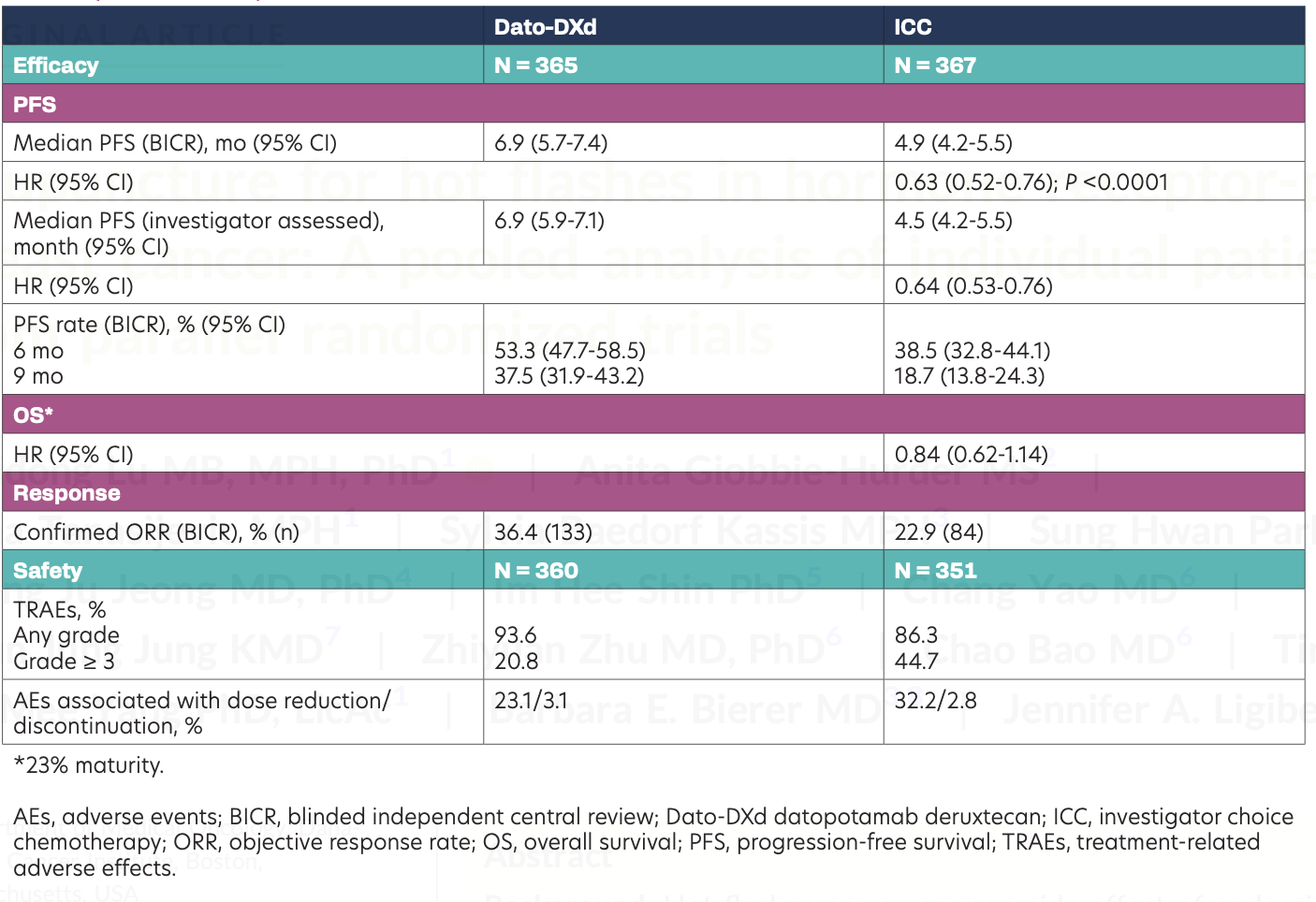
A total of 732 patients were randomized (Dato-DXd: n = 365; ICC: n = 367). Median age was 56 (range, 29-86) and 54 (range, 28-86) years in the Dato-DXd and ICC groups, respectively. At data cutoff (July 17, 2023), 93 and 39 patients in the Dato-DXd and ICC groups, respectively, were ongoing treatment. Results are shown in the Table. Patients receiving Dato-DXd had significantly improved PFS vs ICC (HR, 0.63; 95% CI, 0.52-0.76; P < .0001). OS data were not mature; a trend for improvement favoring Dato-DXd was observed. Patients receiving Dato-DXd had lower rates of grade 3 or higher treatment-related adverse effects (TRAEs) and dose reductions vs ICC.
Conclusions
TROPION-Breast01 met the primary end point of PFS; the study continues to the final OS. Patients receiving Dato-DXd had statistically significant and clinically meaningful improvement in PFS compared with ICC, along with a favorable and manageable safety profile. Results support Dato-DXd as a novel treatment option for patients with inoperable or metastatic HR+/HER2– breast cancer who have received 1 to 2 prior lines of CT.

Giredestrant Combo Yields Positive PFS in Subgroups After CDK4/6i in ER+/HER2– Breast Cancer
December 13th 2025“The magnitude of clinical benefit was clinically meaningful and consistent, and was regardless of PIK3CA mutations or alterations in the PIK3CA pathway, duration of prior CDK4/6 inhibitors, including patients who progress within 6 to 12 months, and the choice of prior CDK4/6 inhibitors,” said Hope S. Rugo, MD.