Accelerated Partial-Breast Irradiation: The Current State of Our Knowledge
Accelerated partial-breast irradiation is a shorter, alternative radiation technique for select patients with favorable early-stage breast cancer. We review here the different modalities of delivery and discuss the possible benefits and harms associated with these treatments.
Breast-conserving therapy consisting of segmental mastectomy followed by whole-breast irradiation (WBI) has become widely accepted as an alternative to mastectomy as a treatment for women with early-stage breast cancer. Accelerated partial-breast irradiation (APBI) is a shorter, alternative radiation technique for select patients with favorable early-stage breast cancer. We review here the different modalities of APBI delivery and discuss the possible benefits and harms associated with these treatments.
For women with early-stage breast cancer, breast-conserving therapy (BCT) is a well-established treatment option, comparable to total mastectomy with regard to rates of local-regional control and overall survival, yet enabling patients to maintain their breasts with acceptable cosmesis.[1] Based on the initial trials conducted in the 1970s and 1980s, BCT has typically involved segmental mastectomy and some degree of lymph node surgery, followed by whole-breast irradiation (WBI) over the course of 5 weeks, with or without a “boost” treatment to the tumor bed. WBI is a relatively well-tolerated treatment that typically has resulted in good long-term cosmetic results, with low rates of treatment-associated morbidity. A meta-analysis published by the Early Breast Cancer Trialists’ Collaborative Group has demonstrated that the addition of radiation therapy (RT) to breast-conserving surgery has been found to improve not only local-regional control, but also long-term overall survival.[2] Changing conventional radiation treatment to target only part of the breast must be scrutinized not only because of potential changes in local control but also on account of possible differences in overall survival when patients are followed for a long period of time.
FIGURE 1
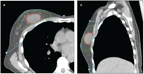
Axial (A) and Sagittal (B) Images Showing the Target Areas in Breast Radiation.
The Rationale Behind APBI
Accelerated partial-breast irradiation (APBI) is an approach in which only the area of the breast where the tumor was initially located is targeted with radiation, with treatment typically delivered over 1 to 15 days. While WBI typically encompasses the breast parenchyma, chest wall musculature, ribs, and approximately 60% of the level I and II lymph nodes, APBI focuses radiation on a 1- to 2-cm margin of tissue surrounding the lumpectomy cavity (Figure 1). The rationale for irradiating only part of the breast stems from the observation that the majority of breast cancer recurrences following lumpectomy alone occur adjacent to the lumpectomy cavity, as evidenced by data from three randomized trials comparing treatment with lumpectomy alone with lumpectomy followed by WBI.[1,3,4] Indeed, in these studies, the incidence of treatment failures elsewhere in the breast, far from the initial tumor bed, occurred at the same rate in patients who received WBI as in those who did not, implying that the main local benefit of WBI is in the area of the tumor bed. Irradiating less of the breast would reduce dose to uninvolved breast tissue, the lungs, the ribs, the chest wall musculature, and the heart, all of which might reduce the risk of late complications.[5]
Compared with a 5- to 6-week WBI treatment, it has been argued that the shorter course of APBI will improve patient satisfaction and overall quality of life, potentially minimizing the psychological and physical strain associated with radiation treatment.[6,7] For those patients who live far from a radiation oncology facility or who suffer from multiple medical comorbidities, a shorter treatment might improve compliance with completing the prescribed course of radiation and/or minimize the election of mastectomy over BCT.[8-12] Some recent analyses have highlighted that certain modalities of delivering APBI might also decrease the overall financial cost of treatment compared with WBI.[13-16]
APBI: Techniques and Clinical Outcomes
Multiple distinct treatment modalities have been used to deliver APBI, including brachytherapy, intraoperative techniques (x-rays, electrons), and external radiation with photons and/or protons. Each modality offers advantages and disadvantages, depending on patient anatomy and preference, as well as the resources available at a particular radiation oncology facility. Ideally, each patient is assessed for the technique that will best meet her individual needs.
Multicatheter interstitial brachytherapy
Multicatheter interstitial brachytherapy (MIB) is the APBI technique with the longest follow-up and the most mature data to support its use. MIB involves the placement of approximately 10 to 20 catheters in the breast tissue surrounding the lumpectomy cavity. This procedure can be performed under local anesthesia and is done only after final pathology results from the initial surgery have returned. Low or high dose rate radioactive sources are temporarily loaded into the catheters in a way that allows dose delivery to the tumor bed and a margin of 1 to 2 cm. Because of the properties of the radioactive sources used, MIB allows for highly conformal treatment, with near complete sparing of surrounding normal tissues.
The Radiation Therapy Oncology Group (RTOG) conducted a phase II, multi-institutional study, RTOG 95-17, which included 99 patients with early-stage breast cancer treated with APBI delivered via low or high dose rate brachytherapy. In this study, the 10-year rate of ipsilateral breast tumor recurrence (IBTR) was 6.2%, and half the recurrences were outside the area of the treatment field.[17] Investigators at the National Institute of Oncology in Hungary have presented their 10-year follow-up of a randomized trial of 258 women randomly assigned to APBI or WBI, with APBI delivered either via MIB high dose rate (HDR) brachytherapy or external electron beam irradiation. The rates of recurrence were not significantly different between the two arms (5.9% for APBI vs 5.1% for WBI), and patients treated with APBI were found to have higher rates of good-to-excellent cosmesis (81% vs 63%, P = .009).[18]
Detractors of this technique point to its invasiveness and the associated risk of infection and scarring. Among the prospective data published on interstitial brachytherapy used to deliver APBI, the rates of infection range from 0% to 11%, with the majority of studies citing rates < 5%.[19-22] Additionally, MIB requires a high degree of expertise on the part of both the physician and the physics staff, which is the primary reason it has not gained greater traction.
Single-entry intracavitary brachytherapy catheters
In response to growing clinical interest in APBI and the desire for a more easily implemented technique, intracavitary, single-entry brachytherapy catheters emerged as a way to deliver APBI using brachytherapy via an approach that is simpler for physicians and physicists and less traumatic for patients. A catheter is inserted through a puncture site in the breast and positioned within the lumpectomy cavity, typically under ultrasound guidance after surgery has been completed. At the completion of treatment, the catheter is removed with or without local anesthetic in the office. Unlike with MIB, early, single-lumen versions of the catheters used in single-entry brachytherapy were restricted to patients with specific geometric characteristics, ie, nonsuperficial surgical cavities. More recent multiple-lumen devices allow greater flexibility in treatment planning, enabling both better coverage of the target tissue at risk and decreased dose to the nearby rib and skin.[23]
Since the US Food and Drug Administration (FDA) approved the original single-lumen MammoSite catheter in 2002, the use of brachytherapy is believed to have increased dramatically. A study of Medicare billing claims among women treated with BCT estimated that the use of brachytherapy as a component of oncologic care increased in incidence from less than 1% of cases in 2001 to 10% of cases by 2006.[24] The American Society of Breast Surgeons has prospectively followed a cohort of 1,440 women treated with the single-lumen MammoSite catheter. This group has reported a 5-year actuarial IBTR rate of 3.8%.[25] Of the patients in this study, 90.6% had good-to-excellent cosmesis, 13% developed symptomatic seromas, 9.5% developed an infection, and 2.3% developed fat necrosis, results comparable to those seen with MIB.[26] More concerning have been the toxicities of brachytherapy-based APBI compared to those of WBI in population-based studies. Smith et al conducted a retrospective population-based cohort study of 92,735 women aged 67 or older with invasive breast cancer diagnosed between 2003 and 2007, treated with BCT, and followed through 2008.[27] The study compared 6,952 women treated with brachytherapy vs 85,783 treated with WBI. The 5-year incidence of subsequent mastectomy was slightly higher among women treated with brachytherapy (4.0% vs 2.2%). It remains uncertain whether this difference in mastectomy rates was due to local recurrence (no data were available regarding the rate of local recurrence) or to toxicities that required surgical treatment, or to other unknown causes; it is also unclear whether this incremental difference negatively offsets the convenience of accelerated treatment. Brachytherapy was also associated with a higher rate of infectious and noninfectious complications, including breast pain, fat necrosis, and rib fracture. A follow-up study by Presley et al reported on 29,648 Medicare beneficiaries aged 66 to 94 treated with BCT between 2008 and 2009.[28] There was a significantly higher rate of wound and skin complications among the 15.8% of women who received brachytherapy instead of WBI (adjusted rate of 33.7% vs 16.8%, P < .001), a rate that far exceeds anything published in the prospective literature. Notably, there was significant geographic variability in the frequency of treatment delivery with brachytherapy nationally, a finding also demonstrated in a Surveillance, Epidemiology, and End Results (SEER) database study looking at national trends.[29] This finding implies that nonclinical factors influenced the implementation of brachytherapy-based APBI. These studies have the benefit of providing information about a broad cross section of patients treated throughout the United States, with large patient numbers. Deficits of these studies include, in most, a lack of information about clinical and tumor characteristics of the patients to whom treatment was given, making it difficult to ascertain whether treatment was given in a way that most would deem appropriate.
External beam irradiation
External beam irradiation, which can be delivered noninvasively, using the standard linear accelerator found in all radiation oncology facilities, is another way to deliver APBI.[30] The RTOG published initial efficacy results from RTOG 03-19, a phase II, multi-institutional study using external beam irradiation to deliver APBI to 58 patients who have stage I/II breast cancer with tumors less than 3 cm in size and three or fewer involved lymph nodes, following segmental mastectomy with negative margins.[31] The authors reported a 4-year IBTR rate of 6%, with two-thirds of these recurrences within the treatment field; 30% of patients developed grade 1/2 pain, and 4% (2 patients) developed grade 3 skin toxicities.
The preliminary toxicity results of a large Canadian cooperative group trial were presented at the annual meeting of the American Society of Radiation Oncology (ASTRO) in 2012.[32] This trial randomly assigned 2,135 women aged 40 or older with early-stage breast cancer following BCT to either WBI or external beam, 3-dimensional conformal APBI. Cosmesis was assessed by the patient, physician, and a nurse, all of whom found the cosmesis at 3 years following completion of treatment was worse in the group treated with APBI (ie, by nursing assessment, poor-to-fair cosmesis in 32% of patients treated with APBI vs 19% of patients treated with WBI, P < .0001). Grade 1/2 late radiation toxicities were also worse in the group treated with APBI. It remains uncertain what the cause of this toxicity was and whether this finding will be replicated in the large pending trials comparing APBI with WBI; nonetheless, these findings should be of concern to those considering offering this treatment to patients.
Intraoperative radiotherapy
Single-fraction, intraoperative radiation therapy has been investigated primarily in Europe as a way to deliver either monotherapy or boost radiation treatment at the time of lumpectomy.[33,34] The chief advantage of intraoperative radiotherapy is delivery of radiation precisely to the operative bed at the time of surgery, without any prolonged treatment or additional invasive procedures. However, this treatment is delivered without image guidance, and concerns have been raised that radiobiologic assumptions about dose escalation break down at such high doses per fraction.[35] Also, margin status is not known at the time of surgery, and concerning pathology requires subsequent retreatment with additional radiation.
The Targeted Intra-Operative Radiotherapy (TARGIT-A) trial has published randomized controlled data on 2,232 patients in whom intraoperative kilovotage (kV) x-rays were used to target 1 cm away from the lumpectomy cavity, compared with WBI with or without a boost.[33] Kilovoltage x-rays are considered to be more radiobiologically effective than the megavoltage x-rays used in traditional external beam RT; different forms of RT have been found in the laboratory to result in differential degrees of effect on such biologic endpoints as tumor cell kill or even damage to normal tissues, such as ribs.[36] However, debate remains as to whether the dose prescribed is sufficient to provide tumor eradication that would be equivalent to what is provided by WBI or other forms of APBI.[35] At 4 years, the rate of in-breast recurrence in TARGIT-A participants was equal in the two arms (1%).
Investigators in Milan recently presented their findings from the Electron Intraoperative Trial (ELIOT), in which 1,305 patients were randomly assigned to receive either WBI with a boost or intraoperative treatment with electrons.[34] At 5 years, 3% of patients receiving APBI had a failure in the same quadrant as the primary tumor, compared with 1% of those treated with WBI. Breast failure elsewhere was 2% with APBI and 0% with WBI. With regard to toxicities, overall the patients treated with APBI did better than those treated with WBI, according to data presented at the 2012 World Congress of Brachytherapy; however, the detailed data have not yet been presented.[34]
Protons
Protons offer another external modality for the delivery of radiation therapy that is noninvasive and that provides a sharper dose fall-off than photon- and electron-based external beam irradiation. The delivery of proton treatment is available at approximately a dozen sites internationally. Initial dosimetric analyses comparing protons to photon-based APBI demonstrated that the former provided comparable coverage of the target tissue at risk while decreasing the radiation dose delivered to the nontarget breast, lung, and heart.[37] Unfortunately, the initial clinical publication from Massachusetts General Hospital reporting on 20 patients who were being followed revealed a relatively high rate of toxicities (22% of patients with moderate-to-severe moist desquamation at 6 to 8 weeks, 3 patients with telangiectasias, and 1 rib fracture, with a median follow-up of 12 months). In spite of this, 95% of patients reported satisfaction with their treatment.[38] Investigators at Loma Linda have published their 4-year follow-up of a phase II study of 50 patients treated with APBI using protons. They reported a total of only 3 late grade 1 telangiectasias, and acute toxicities were grade 1/2 in half of the patients.[39] It is unclear what caused the difference between these two studies, although there were differences in the beam arrangements used by these groups. Although facilities with the capability of delivering protons are currently limited in number, it is anticipated that many more such centers will be opening in the near future.
How to Compare These Modalities?
APBI vs WBI
WBI implicitly treats the breast as if every part were equally at risk for recurrence. A radiation dose appropriate for presumed microscopic disease is prescribed, and radiation “hot spots” in excess of this are considered undesirable due to toxicity concerns. During treatment planning, efforts are made to keep the radiation dose throughout the breast homogeneous to about ±10% or better.
On the other hand, WBI is commonly followed by a radiation "boost" to the vicinity of the surgical cavity. In effect, this is a deliberate “hot spot” that increases the radiation dose in this region to levels comparable to those used to control gross disease. The addition of a boost improves local control by approximately 40%, although at the cost of tripling the incidence of fibrosis (with a 10-year rate of 4.4% with the boost vs 1.6% without in the European Organisation for Research and Treatment of Cancer [EORTC] 22881-10882 trial).[40] The boost is, in a way, an admission that the assumption of equal recurrence risk across the entire breast is flawed. However, the question of what tissue volume should receive boost radiation is open, and practices vary. Most boost targets are defined by a margin around the surgical cavity, and the literature contains reports of margins ranging from 0 to 2.5 cm. From a planning and treatment delivery standpoint, a photon boost to the surgical cavity + 2 cm can be virtually indistinguishable from external beam APBI.
FIGURE 2
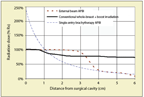
Comparison of the Dose Distributions of Whole-Breast Irradiation With a Boost, External Beam APBI, and Single-Entry Brachytherapy APBI.
While external beam approaches aim for dose uniformity within irradiated regions, brachytherapy doses are decidedly not uniform. When using a balloon catheter, radiation doses at the cavity can be more than double those seen in external beam treatment, and the dose falls off continuously with distance (Figure 2). Rather than treat the breast as being at one uniform risk level for recurrence (WBI) or two uniform risk levels (WBI + boost, external beam ABPI), brachytherapy, especially single-entry, essentially treats risk as continuously decreasing with distance from the cavity. It would be useful to map the actual spatial dependence of local recurrence risk against the different dose profiles shown in Figure 2. The result might well support a more nuanced approach to breast radiotherapy than an all-or-nothing, WBI vs APBI dichotomy. Unfortunately, at this time, applicable pathological studies are scarce.
Although “partial-breast” irradiation has been cited as the factor that permits “accelerated” irradiation, radiobiological calculation shows that the doses commonly delivered by APBI are actually equivalent to those of WBI in terms of both expected local control and toxicity.[41] Therefore, the “partial” and “accelerated” aspects of APBI can be viewed as separate rather than going hand-in-hand. Accelerated WBI, commonly delivered over 3 weeks, is becoming more and more widely adopted. On the other hand, it has been argued that partial-breast irradiation should be less accelerated. Given the dose equivalence, it has been unclear why APBI, which irradiates less of the breast, has been reported to have, at best, toxicity equivalent to WBI. It is the twice-daily treatment that allows common forms of APBI to be delivered in 1 week as opposed to multiple weeks, and it has been suggested that using a once-daily treatment might resolve this issue.[42]
APBI Treatment Modalities
External beam APBI treats larger volumes of tissue than does brachytherapy, and the impact of this is unclear.[43] Historical mastectomy series attempting to answer the question of the extent of microscopic residual disease following a simulated lumpectomy have limited utility in the modern era, in which APBI is offered to treat relatively smaller tumors.[44,45] At least two more modern pathologic studies involving re-excision following wide local excision for early-stage breast cancer have found that in more than 90% of cases an additional resection margin of 1 cm encompassed any remaining microscopic residual disease.[46,47] In addition to target volume, different APBI modalities typically involve different radiation dose prescriptions. Radiobiologic calculations have been made in an attempt to define the degree to which the distinct methods of delivering APBI are equivalent to WBI; however, most of these have been done post hoc and have not been based on any endpoints of local control.[41] One study demonstrated that differences in dose homogeneity suggest that external beam dose prescriptions should be slightly higher than MIB brachytherapy prescriptions (as is the usual practice) to be equally effective.[48] There continue to be debates about the equivalency of the doses prescribed with intraoperative x-ray or electron-based radiation compared with the other modalities of APBI.[35]
Even when comparing relatively similar brachytherapy techniques for delivering APBI, there remains uncertainty regarding the appropriate dosimetric criteria needed to minimize the risk of such toxicities as fat necrosis. In the initial clinical experiences with interstitial catheter-based brachytherapy, symptomatic fat necrosis was reported in anywhere from 2% to 21% of cases.[21,22,49] The current guidelines in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/RTOG 0413 protocol regarding the volume of higher doses of radiation permissible on study are based on earlier dosimetric analyses from Tufts University and Virginia Commonwealth University that correlated larger volumes of high doses of radiation in the breast with the development of fat necrosis.[50] It is difficult to know how to translate these dosimetric constraints from one brachytherapy modality to another, which is important given that balloon and strut-based single-entry brachytherapy catheters yield significantly different dose distributions. Additionally, tumor proximity to such structures as the ribs, chest wall musculature, and skin may portend a different outcome with APBI, given the relatively steep dose gradient in the area targeted.
Quality of Life Benefits
We have seen in our clinical practices that patients who receive APBI express deep gratitude at being offered the option of a more condensed treatment. Surprisingly, there have been limited published data obtained from surveying women in order to better understand their feelings regarding the convenience of finishing radiation treatment within 1 week with twice-daily treatments compared with daily treatment over 3 to 6 weeks[51]; thus, it remains difficult to quantify this benefit, which results in physicians relying on anecdotal stories of individual patients they have treated.
One of the original rationales behind APBI was that it would enable more women to have access to BCT-women for whom it would have been too difficult to undergo a more prolonged course of WBI and who might otherwise have opted to pursue mastectomy for this reason alone. To our knowledge, there has not yet been a study documenting the trends in utilization of BCT vs mastectomy since APBI has become more broadly used, and we would welcome research toward this end. The population-based studies that have been published have found that brachytherapy-based APBI has been most prevalent in metropolitan areas or their environs.[52] This difference in treatment delivery may be due to both patient preference and available resources, as HDR brachytherapy and the requisite physicist and physician staffing may be less available in more rural areas. Thus far, there has been no published documentation on the trends in usage of external beam–based APBI in such locales.
The Costs of APBI
The overall clinical cost of APBI, to both patients and society, has not been fully quantified. A shorter course of external beam APBI clearly costs less than a protracted course of external beam WBI in terms of the amount billed to the patient, the number of healthcare resources used, and the financial cost to the patient in terms of time away from work and family.[13,14,16] However, since the inception of single-entry brachytherapy catheters, patterns of reimbursement have changed significantly. With this technique, typically there is a financial charge for the catheter and the placement of the catheter by the surgeon. In addition, this treatment technique is significantly more time-intensive to the physician and medical physicist involved in the case, and reimbursement has varied over time, with the amount paid increasing in accordance with the number of lumens treated, a reflection of the greater complexity of the case. A few publications analyzing the cost of APBI compared with WBI have resulted in mixed findings.[14-16] In general, these studies have shown that external beam–based APBI is significantly less expensive than standard fractionation WBI over 5 to 6 weeks. However, the comparison of brachytherapy-based APBI to WBI with a boost is more complicated, and the costs of these two approaches appear generally comparable; however, such things as intensity-modulated radiotherapy (IMRT) treatment plans and the brachytherapy catheter itself add additional costs. Proton-based treatment in general has been found to be more costly than other more traditional forms of radiation therapy, although the cost of proton-based APBI and that of traditional WBI have not yet been directly compared.
Most APBI regimens require twice-daily treatment, with a minimum 6-hour time interval between treatments, a regime for which many patients require time off from work. However, these patients typically finish treatment within 1 week instead of the traditional 6 weeks of standard WBI, which means a less prolonged interruption of normal work and home activities. This difference might be minimized as hypofractionated WBI continues to increase in use, allowing treatment of the whole breast in 3 to 4 weeks.
TABLE 1

Published Consensus Statement Criteria for the Delivery of Accelerated Partial-Breast Irradiation Off Protocol
Brachytherapy-based APBI is more labor-intensive from the perspective of the breast surgeon, radiation oncologist, and medical physicist. This treatment requires close communication and synchronization of scheduling between the breast surgeon and the radiation oncologist, which can be challenging in a busy clinical practice. In addition, the Nuclear Regulatory Committee requires the attendance of both a radiation oncologist and a medical physicist at the delivery of each fraction of radiation for brachytherapy and intraoperative treatment, in addition to daily quality assurance by the medical physicist. By our estimates, this adds at least 10 extra hours worked per patient compared with external beam–based treatment.
Guidelines for Treatment
Beginning in 2007, four separate oncologic societies have published guidelines to assist physicians in selecting patients for APBI offered off protocol (Table 1). ASTRO organized a task force that published the most detailed set of guidelines regarding suitability to receive APBI off protocol. This group recommended that women be classified as “suitable” for APBI treatment if they are age 60 or older and have invasive ductal carcinoma (or other favorable histology) ≤ 2 cm; estrogen receptor–positive; unicentric and unifocal; lymph node–negative; with negative margins (≥ 2 mm); and with no extensive intraductal component (EIC), lymphovascular space invasion (LVSI), or neoadjuvant therapy.[53] The Groupe Europen de Curiethrapie and European Society for Therapeutic Radiology and Oncology (GEC-ESTRO), the American Society of Breast Surgeons, and the American Brachytherapy Society have all published their own sets of guidelines.[54-56] These four sets of guidelines were based on older published studies and the inclusion criteria used therein, coupled with the judgment of experienced breast radiation oncologists.
TABLE 2

Closed Randomized Trials Comparing Accelerated Partial-Breast Irradiation and Whole-Breast Irradiation
It is worth noting that none of the criteria defining those patients considered to be ill suited to receive APBI were based on published reports of worse outcomes in those patient subsets. Indeed, most of the factors listed in the guidelines are related to general risk factors for the recurrence of breast cancer in the setting of BCT. To our knowledge, none of the criteria listed are known to directly correlate with a patient’s risk of having a higher rate of recurrence if she receives radiation to the whole breast vs to the lumpectomy cavity only. We posit that additional pathologic data mapping the extent of disease could help better determine suitability to receive APBI.
What impact the consensus statements have had on patient selection for treatment with APBI remains uncertain. Some experts have raised the concern that the verbiage chosen in these publications may be misleading.[57,58] Given that these four organizations have not been able to agree on the most important factors in patient selection for the delivery of APBI, the individual medical practitioner will continue to need to make a decision about how best to apply the literature to a given patient’s clinical scenario. However, we believe the published guidelines do offer some direction to clinicians and serve as a useful reference.
TABLE 3
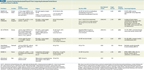
Currently Pending Randomized Phase III Trials Comparing Accelerated Partial-Breast and Whole-Breast Irradiation
APBI: The Future
At least four randomized trials comparing WBI and APBI have been presented thus far, in at least partial form (Table 2); the results regarding toxicity and efficacy have been mixed. In the future, we anticipate the publication of results from at least eight randomized controlled trials comparing WBI and APBI (Table 3). These trials are being conducted in North America and Europe and in total will include over 16,000 women. The largest of these, the NSABP B-39/RTOG 0413 trial, is being conducted in the United States, with trial closure anticipated shortly.[59] This trial will include 4,300 women with early-stage breast cancer randomly assigned to receive either WBI (with or without a tumor bed boost) or APBI. In this study, APBI can be delivered via MIB, single-entry brachytherapy catheter, or 3-dimensional conformal external beam radiation. While the trials now open vary in terms of patient inclusion criteria and how APBI is delivered, we expect that they will provide definitive information on the equivalency of APBI to WBI. However, given the differences in the mode of APBI delivery in these studies, the findings of one may not be applicable to the technique used in another, in terms of either tumor control or toxicity.
Meanwhile, investigators are attempting to answer such questions as whether MRI can be used to better define patients who can be treated with APBI, whether brachytherapy-based APBI can be delivered with fewer total fractions, whether APBI can be delivered preoperatively, and whether pathologic and surgical margin findings can be used for patient-specific target definition in APBI.[60-66]
Conclusion
Given its greater convenience and perceived better associated quality of life, APBI is becoming an increasingly popular alternative to conventional WBI among women with early-stage breast cancer who desire breast conservation. Published data on APBI are limited largely to findings from prospectively collected phase II trials, which have shown high rates of local control in appropriately selected patients. Several sets of guidelines have been published outlining which patients should be considered reasonable candidates for treatment with APBI. We anticipate in the near future the publication of results from a number of randomized controlled trials including over 16,000 women, which should better characterize the efficacy and safety of APBI in comparison to WBI.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-41.
2. Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-16.
3. Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997-1003.
4. Liljegren G, Holmberg L, Bergh J, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326-33.
5. Mukesh M, Harris E, Jena R, et al. Relationship between irradiated breast volume and late normal tissue complications: a systematic review. Radiother Oncol. 2012;104:1-10.
6. Kawase E, Karasawa K, Shimotsu S, et al. Estimation of anxiety and depression in patients with early stage breast cancer before and after radiation therapy. Breast Cancer. 2012;19:147-52.
7. Whelan TJ, Levine M, Julian J, et al. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88:2260-6.
8. Lazovich DA, White E, Thomas DB, Moe RE. Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA. 1991;266:3433-8.
9. Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097-101.
10. Athas WF, Adams-Cameron M, Hunt WC, et al. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92:269-71.
11. Ma AM, Barone J, Wallis AE, et al. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am J Surg. 2008;196:500-4.
12. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145:637-45.
13. Suh WW, Pierce LJ, Vicini FA, Hayman JA. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;62:790-6.
14. Sher DJ, Wittenberg E, Suh WW, et al. Partial-breast irradiation versus whole-breast irradiation for early-stage breast cancer: a cost-effectiveness analysis. Int J Radiat Oncol Biol Phys. 2009;74:440-6.
15. Lanni T, Keisch M, Shah C, et al. A cost comparison analysis of adjuvant radiation therapy techniques after breast-conserving surgery. Breast J. 2013;19:162-7.
16. Shah C, Lanni TB, Saini H, et al. Cost-efficacy of acceleration partial-breast irradiation compared with whole-breast irradiation. Breast Cancer Res Treat. 2013;138:127-35.
17. White JR, Winter KA, Kuske RR, et al. Long-term outcome from RTOG 95-17: a phase I/II study of accelerated partial breast irradiation (APBI) with multicatheter brachytherapy (MCT) following lumpectomy for early-stage breast cancer. J Clin Oncol. 2012;30(Suppl 27):Abstr 147.
18. Polgar C, Major T, Fodor J, et al, eds. Breast-conserving therapy with partial or whole breast RT: 10-year results of the Budapest randomized trial. World Congress of Brachytherapy; 2012; Barcelona, Spain.
19. Hattangadi JA, Powell SN, MacDonald SM, et al. Accelerated partial breast irradiation with low-dose-rate interstitial implant brachytherapy after wide local excision: 12-year outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:791-800.
20. Strnad V, Hildebrandt G, Potter R, et al. Accelerated partial breast irradiation: 5-year results of the German-Austrian multicenter phase II trial using interstitial multicatheter brachytherapy alone after breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2011;80:17-24.
21. Chen PY, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation: a method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;106:991-9.
22. Polgar C, Sulyok Z, Fodor J, et al. Sole brachytherapy of the tumor bed after conservative surgery for T1 breast cancer: five-year results of a phase I-II study and initial findings of a randomized phase III trial. J Surg Oncol. 2002;80:121-8; discussion 29.
23. Arthur DW, Vicini FA, Todor DA, et al. Improvements in critical dosimetric endpoints using the Contura multilumen balloon breast brachytherapy catheter to deliver accelerated partial breast irradiation: preliminary dosimetric findings of a phase IV trial. Int J Radiat Oncol Biol Phys. 2011;79:26-33.
24. Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for accelerated partial-breast irradiation: a rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;29:157-65.
25. Vicini F, Beitsch P, Quiet C, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808-17.
26. Khan AJ, Arthur D, Vicini F, et al. Six-year analysis of treatment-related toxicities in patients treated with accelerated partial breast irradiation on the American Society of Breast Surgeons MammoSite Breast Brachytherapy registry trial. Ann Surg Oncol. 2012;19:1477-83.
27. Smith GL, Xu Y, Buchholz TA, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827-37.
28. Presley CJ, Soulos PR, Herrin J, et al. Patterns of use and short-term complications of breast brachytherapy in the national Medicare population from 2008-2009. J Clin Oncol. 2012;30:4302-7.
29. Abbott AM, Habermann EB, Tuttle TM. Trends in the use of implantable accelerated partial breast irradiation therapy for early stage breast cancer in the United States. Cancer. 2011;117:3305-10.
30. Baglan KL, Sharpe MB, Jaffray D, et al. Accelerated partial breast irradiation using 3D conformal radiation therapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2003;55:302-11.
31. Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/ II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120-7.
32. Whelan TJ, Olivotto I, Parpia S, et al. Interim toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation (APBI) using 3D conformal external beam radiation therapy (3D CRT). Int J Radiat Oncol Biol Phys. 2013;85:21-22.
33. Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91-102.
34. Orecchia R. ELIOT Trials in Milan: Results. World Congress of Brachytherapy; Barcelona, Spain. 2012.
35. Khan AJ, Dale RG, Arthur DW, et al. Ultrashort courses of adjuvant breast radiotherapy: wave of the future or a fool's errand? Cancer. 2012;118:1962-70.
36. Vaidya JS, Tobias JS, Baum M, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol. 2004;5:165-73.
37. Taghian AG, Kozak KR, Katz A, et al. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int J Radiat Oncol Biol Phys. 2006;65:1404-10.
38. Kozak KR, Smith BL, Adams J, et al. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys. 2006;66:691-8.
39. Bush DA, Slater JD, Garberoglio C, et al. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin Breast Cancer. 2011;11:241-5.
40. Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007; 25:3259-65.
41. Rosenstein BS, Lymberis SC, Formenti SC. Biologic comparison of partial breast irradiation protocols. Int J Radiat Oncol Biol Phys. 2004;60:1393-404.
42. Bentzen SM, Yarnold JR. Reports of unexpected late side effects of accelerated partial breast irradiation-radiobiological considerations. Int J Radiat Oncol Biol Phys. 2010;77:969-73.
43. Shaitelman SF, Vicini FA, Grills IS, et al. Differences in effective target volume between various techniques of accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2012;82:30-6.
44. Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979-90.
45. Faverly DR, Hendriks JH, Holland R. Breast carcinomas of limited extent: frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer. 2001;91:647-59.
46. Vicini FA, Kestin LL, Goldstein NS. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: a pathologic analysis. Int J Radiat Oncol Biol Phys. 2004;60:722-30.
47. Nguyen BT, Deb S, Fox S, et al. A prospective pathologic study to define the clinical target volume for partial breast radiation therapy in women with early breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:1116-22.
48. Cuttino LW, Todor D, Pacyna L, et al. Three-dimensional conformal external beam radiotherapy (3D-CRT) for accelerated partial breast irradiation (APBI): what is the correct prescription dose? Am J Clin Oncol. 2006;29:474-8.
49. Wazer DE, Berle L, Graham R, et al. Preliminary results of a phase I/II study of HDR brachytherapy alone for T1/T2 breast cancer. Int J Radiat Oncol Biol Phys. 2002;53:889-97.
50. Wazer DE, Lowther D, Boyle T, et al. Clinically evident fat necrosis in women treated with high-dose-rate brachytherapy alone for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2001;50:107-11.
51. Albuquerque K, Tell D, Lobo P, et al. Impact of partial versus whole breast radiation therapy on fatigue, perceived stress, quality of life and natural killer cell activity in women with breast cancer. BMC Cancer. 2012;12:251.
52. Hattangadi J, Taback N, Neville BA, et al. Accelerated partial breast irradiation using brachytherapy for breast cancer: patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012;104:1-13.
53. Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009;74:987-1001.
54. Polgar C, Van Limbergen E, Potter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol. 94:264-73.
55. Board of Directors ASoBS. Consenus statement for accelerated partial breast irradiation. 2011. Available from: https://www.breastsurgeons.org/statements/PDF_Statements/APBI.pdf. Accessed March 26, 2013.
56. Breast Brachytherapy Task Group, ABS. ABS Breast Brachytherapy Task Group. 2007. Available from: http://www.americanbrachytherapy.org/guidelines/abs_breast_brachytherapy_taskgroup.pdf. Accessed March 26, 2013.
57. Vicini F, Arthur D, Wazer D, et al. Limitations of the American Society of Therapeutic Radiology and Oncology Consensus Panel guidelines on the use of accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:977-84.
58. Shaitelman SF, Vicini FA, Beitsch P, et al. Five-year outcome of patients classified using the American Society for Radiation Oncology consensus statement guidelines for the application of accelerated partial breast irradiation: an analysis of patients treated on the American Society of Breast Surgeons MammoSite Registry Trial. Cancer. 2010;116:4677-85.
59. Wolmark N, Curran W. NSABP Protocol B-39. RTOG Protocol 0413. A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer. 2011. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00103181. Accessed March 26, 2013.
60. Schmitz AC, Pengel KE, Loo CE, et al. Pre-treatment imaging and pathology characteristics of invasive breast cancers of limited extent: potential relevance for MRI-guided localized therapy. Radiother Oncol. 2012;104:11-8.
61. Khan AJ. Safety study for short-course accelerated, hypofractionated partial breast radiotherapy (APBI) in women with early stage breast cancer using the Contura MLB. 2010. Available from: http://clinicaltrials.gov/show/NCT01072838. Accessed March 26, 2013.
62. Palta M, Yoo S, Adamson JD, et al. Preoperative single fraction partial breast radiotherapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;82:37-42.
63. Kirby AM, Evans PM, Nerurkar AY, et al. How does knowledge of three-dimensional excision margins following breast conservation surgery impact upon clinical target volume definition for partial-breast radiotherapy? Radiother Oncol. 2010;94:292-9.
64. Hanbeukers B, Borger J, van den Ende P, et al. Customized computed tomography-based boost volumes in breast-conserving therapy: use of three-dimensional histologic information for clinical target volume margins. Int J Radiat Oncol Biol Phys. 2009;75: 757-63.
65. Stroom J, Schlief A, Alderliesten T, et al. Using histopathology breast cancer data to reduce clinical target volume margins at radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:898-905.
66. Dorn PL, Al-Hallaq HA, Haq F, et al. A prospective st6dy of the utility of magnetic resonance imaging in determining candidacy for partial breast irradiation. Int J Radiat Oncol Biol Phys. 2013;85:615-22.
67. Rampinelli C, Bellomi M, Ivaldi GB, et al. Assessment of pulmonary fibrosis after radiotherapy (RT) in breast conserving surgery: comparison between conventional external beam RT (EBRT) and intraoperative RT with electrons (ELIOT). Technol Cancer Res Treat. 2011;10:323-9.
68. Coles C, Yarnold J. The IMPORT trials are launched (September 2006). Clin Oncol (R Coll Radiol). 2006;18:587-90.
69. Yarnold J. Randomised trial testing intensity modulated and partial organ radiotherapy after breast conservation surgery for early breast cancer. 2008 [updated January 6, 2011]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00814567. Accessed March 26, 2013.
70. Strnad V, Polgár, C. Interstitial brachytherapy alone versus external beam radiation therapy after breast conserving surgery for low risk invasive carcinoma and low risk duct carcinoma in-situ (DCIS) of the female breast. Available from: http://www.apbi.uni-erlangen.de/outline/outline.html. Accessed March 26, 2013.
71. Frezza G, Bertoni, F. IRMA. Breast cancer with low risk of local recurrence: partial and accelerated radiation with three-dimensional conformal radiotherapy (3DCRT) vs. standard radiotherapy after conserving surgery (phase III study). 2007. Available from: http://groups.eortc.be/radio/res/irma/synopsis_trial_irma1.pdf. Accessed March 26, 2013.
72. Danish Breast Cancer Cooperative Group. Partial breast versus whole breast irradiation in elderly women operated on for early breast cancer. 2011. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00892814. Accessed March 26, 2013.
73. Belkacemi Y, Lartigau, E. Standard or hypofractionated radiotherapy versus accelerated partial breast irradiation (APBI) for breast cancer (SHARE). 2011. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01247233. Accessed March 26, 2013.
74. Livi L, Buonamici FB, Simontacchi G, et al. Accelerated partial breast irradiation with IMRT: new technical approach and interim analysis of acute toxicity in a phase III randomized clinical trial. Int J Radiat Oncol Biol Phys. 77:509-15.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.