Recurrent Epithelial Ovarian Cancer: An Update on Treatment
Emerging therapies in the management of ovarian cancer have resulted in a shift in paradigm, including in the appropriate time to institute therapy, and in the selection of therapy. This review focuses on chemotherapy and emerging biologic agents that present a therapeutic option for patients with recurrent ovarian cancer.
Table 1: The Platinum-Free Interval as a Predictor of Future Response to Platinum-Based Therapy
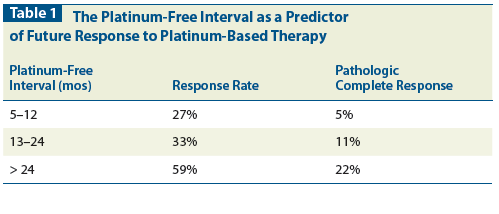
Table 2: Phase III Trials in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer
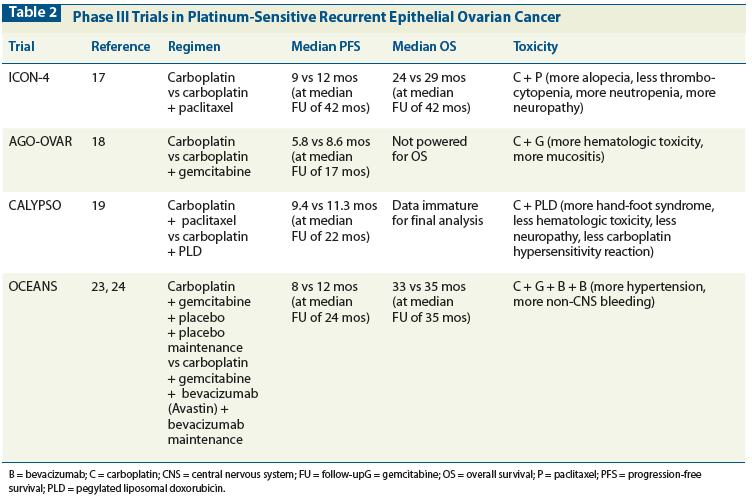
Table 3: Single-Agent Treatment Options in Recurrent Ovarian Cancer
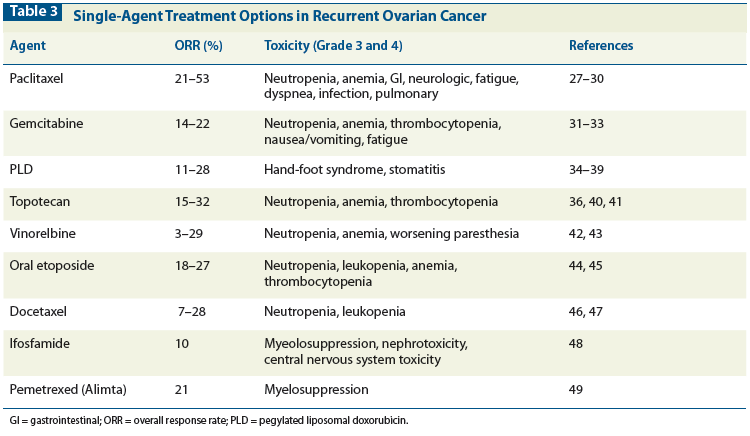
Table 4: Dosing and Scheduling Regimen Options for Paclitaxel
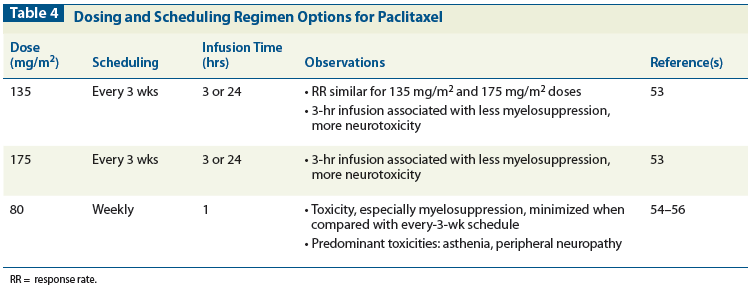
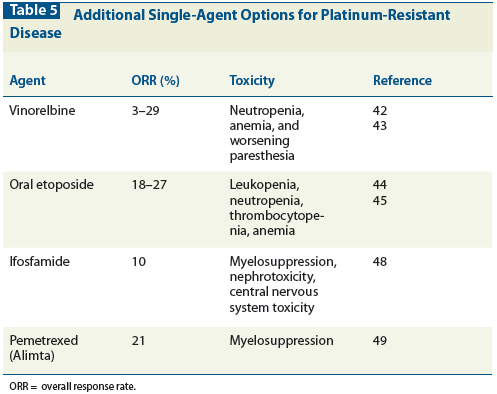
Table 5: Additional Single-Agent Options for Platinum-Resistant Disease
An estimated 85% of patients with epithelial ovarian cancer who achieve a full remission following first-line therapy will develop recurrent disease. Although each subsequent line of therapy is characterized by shorter disease-free intervals, median survival for these patients ranges from 12 months to 24 months. Emerging therapies in the management of ovarian cancer have resulted in a shift in paradigm, including in the appropriate time to institute therapy, and in the selection of therapy. This review focuses on chemotherapy and emerging biologic agents that present a therapeutic option for patients with recurrent ovarian cancer.
Introduction
An estimated 75% of women with ovarian cancer present with advanced-stage (III or IV) disease. For patients with low residual disease (all lesions < 1 cm in size following surgical debulking), the risk for recurrence after completion of primary therapy is 60% to 70%; however, for women with large-volume residual disease, the risk is estimated at 80% to 85%.[1]
We conducted a systematic review using the MEDLINE database, PubMed, EMBASE, and the Cochrane Library. The search was restricted to English-language articles published from 1979 through July 2012. Priority was given to articles reporting original research, in particular randomized controlled trials, although review articles and commentaries also were consulted. Recent abstracts of research presented at symposia and scientific conferences were also considered.
The selection of therapy for women with recurrent disease is in large part determined by response to first-line therapy. Specifically, recurrent ovarian cancer has been dichotomized to either platinum-sensitive (progression-free interval [PFI] > 6 months) or platinum-resistant (PFI ≤ 6 months) disease, with PFI predicting the expected response rate (RR) and duration of response.[2-5] Irrespective of treatment selected, recurrent ovarian cancer remains incurable. The goals of therapy should focus on palliation of cancer-related symptoms, prolongation of life, and optimization of quality of life. This review will focus on the medical treatment, specifically chemotherapy options, available for women with recurrent ovarian cancer.
Role of Timing in the Treatment of Recurrent Ovarian Cancer
Recurrent ovarian cancer can be heralded by onset of new symptoms, radiologic evidence of recurrent disease, or a rising CA-125 level in an asymptomatic patient. CA-125 elevation may precede radiologic detection or onset of symptoms by several months.[6,7] Although formal definitions of ovarian cancer recurrence and progression have been described, based on both clinical and CA-125 criteria, many patients present with either an asymptomatic, radiologic recurrence or an asymptomatic rise in CA-125 level without a radiographic correlate.[7,8]
Given that recurrent disease is incurable, the goals of therapy outlined earlier should guide decisions about initiation of subsequent therapy.[9] In patients with symptomatic recurrence, immediate institution of treatment may be justified and warranted to palliate cancer-related symptoms. For asymptomatic recurrences (rising CA-125 level, for example), timing of therapy is much more controversial. Those who advocate institution of immediate treatment argue that treating small-volume disease is more likely to succeed in achieving a complete response following early intervention.[10-12] Advocates for delaying treatment emphasize that the goal of therapy is palliation, so that medical intervention should be deferred until symptom onset, given the lack of data to show that early treatment improves survival.[13]
The controversy surrounding the appropriate timing of institution of therapy was addressed in a prospective study of 1,442 women with ovarian cancer. The trial was conducted among patients in full clinical remission, with a normal CA-125 level following completion of primary surgical treatment and platinum-based systemic therapy.[14] Both investigators and patients were blinded. Serum CA-125 levels were checked every 3 months. Women whose CA-125 rose to a level twice above the upper limit of normal and remained asymptomatic (n = 527) were randomized to either immediate treatment or treatment when clinical or symptomatic recurrence occurred. Women randomized to immediate therapy initiated chemotherapy a median of 5 months earlier than those who initiated therapy with the onset of symptoms. Survival and remission duration were comparable between the two arms at 57-month follow-up. However, quality of life was worse for the women undergoing immediate treatment. The investigators concluded that early institution of second-line therapy did not benefit patients, advocating for treatment to be delayed until symptoms develop or patients have signs of recurrent disease.
Platinum-Sensitive Recurrent Ovarian Cancer
Importance of the platinum-free interval
In general, platinum-sensitive recurrent ovarian cancer is treated with a platinum agent alone or in combination with another agent. When selecting single-agent vs combination therapy, it is important to consider the patient’s performance status, other comorbidites, and previous toxicities with other therapies. If combination therapy is elected, then the specific combination regimen chosen should also take these factors into account, to optimize outcome and minimize overlapping toxicities. The platinum-free interval-the time between the last cycle of platinum and evidence of disease progression-is now accepted as an important, significant predictor of response to second-line chemotherapy (see Table 1). Some experts have proposed that early use of nonplatinum agents can prolong the platinum-free interval and optimize subsequent retreatment with platinum.
Treatment selection
A phase II trial randomly assigning patients with platinum-sensitive recurrences to either single-agent paclitaxel or combination therapy with cisplatin, doxorubicin, and cyclophosphamide documented similar overall response rates between the two regimens (45% vs 55%) but showed that the platinum-containing combination was associated with a significant increase in response duration (16 vs 9 months) and median survival (35 vs 26 months).[15] This study is limited by its small sample size, phase II design, and the fact that close to 50% of the patients dropped out of the trial at “cross-over.”[15,16] However, the study results are used to advocate the use of platinum first in the management of women with platinum-sensitive recurrent ovarian cancer.[15,16] The choice of whether to use a single platinum agent or a platinum-containing combination should take into account the individual patient’s specific circumstances, beyond the disease-free interval for patients on platinum therapy. Combination regimens are associated with a longer response rate and PFI.[17-21] The paclitaxel-carboplatin combination, for example, has been shown to lead to a longer overall survival (OS) compared with platinum alone. However, combination therapy is more toxic.[17] Selection of single-agent platinum vs combination platinum-containing regimens should also take into account patients’ performance status and previously encountered chemotherapy toxicities.
Combination regimens
In two parallel randomized phase III trials, 802 women with platinum-sensitive recurrent ovarian cancer were treated with a single-agent platinum vs paclitaxel in combination with a platinum agent. The combination regimen was associated with an improved OS (hazard ratio [HR] = 0.82), a 2-year survival benefit of 7%, and 5 months’ improvement in median survival (29 vs 24 months).[17] The combination regimen had a progression-free survival (PFS) with a HR of 0.76, and an absolute 10% difference in 1-year PFS (50% vs 40%).[17] However, the combination was also associated with a higher incidence of grade 2 to 4 neurologic toxicity (20% vs 1%) and alopecia (86% vs 25%).[17] Importantly, 30% of patients had been treated with platinum alone in the primary setting, for tumors that were taxane-naive at time of their entry into the study.[17]
A second phase III trial of single-agent carboplatin compared with a platinum-containing doublet confirmed the observation that doublets are superior to single agents. In the Intergroup trial of the Arbeitsgemeinschaft Gynaekologische Onkologie-OVAR, the National Cancer Institute of Canada Clinical Trials Group, and the European Organisation for Research and Treatment of Cancer Gynecologic Cancer Group, carboplatin and gemcitabine were associated with a longer PFS vs carboplatin alone. The reported PFS for patients randomized to carboplatin was 5.8 months, compared with 8.6 months for patients treated with carboplatin and gemcitabine, (P = .0031; HR = 0.72; 95% CI, 0.57–0.90).[18] The study was not powered to show an OS difference. The results of this trial resulted in the US Food and Drug Administration approval of combination gemcitabine plus carboplatin in platinum-sensitive second-line ovarian cancer in 2006.[18]
In a phase III trial comparing two doublets, taxol and carboplatin vs carboplatin in combination with pegylated liposomal doxorubicin (PLD [Doxil]), PFS with the latter regimen (11.3 months) was not inferior to the taxane-containing doublet (9.4 months; P < .001; HR = 0.82; 95% CI, 0.72–0.94].[19]
The angiogenesis inhibitor bevacizumab (Avastin) has been studied as a treatment option in both first-line ovarian cancer therapy and in patients with recurrent epithelial ovarian cancer.[20-24] The phase III OCEANS study randomized women with platinum-sensitive recurrent ovarian cancer to carboplatin plus gemcitabine with or without bevacizumab for 10 cycles maximum, followed by bevacizumab alone until disease progression or toxicity.[23,24] Compared with chemotherapy plus placebo, bevacizumab with chemotherapy was associated with an improved PFS (12 months with bevacizumab vs 8 months in the placebo group; HR = 0.48; 95% CI, 0.39–0.61), a higher response rate (79% vs 57%, P < .001) and a higher rate of treatment discontinuation for adverse events (23% vs 5%); the latter included higher rates of serious hypertension (17% vs < 1%), proteinuria > grade 3 (9% vs 1%), and non–central nervous system bleeding (6% vs 1%). Notably, there were no cases of gastrointestinal perforation reported during therapy. Overall survival was similar at 35 months of follow-up (33 vs 35 months).[23,24] The Gynecologic Oncology Group (GOG) is conducting a phase III randomized trial to evaluate the role of carboplatin and paclitaxel, with or without bevacizumab, in platinum-sensitive ovarian cancer. Table 2 summarizes these phase III combination trials in platinum-sensitive recurrent ovarian cancer.
Non–platinum-based combination regimens are another emerging option for patients with platinum-sensitive disease. In a phase III randomized trial of PLD alone vs PLD combined with the marine-derived alkaloid trabectedin (Yondelis), the combination regimen resulted in improved PFS.[25]
Single-agent chemotherapy beyond second-line treatment
There are numerous single-agent therapeutic options or patients with platinum-sensitive ovarian cancer. Table 3 lists some of these chemotherapeutic options and their documented response rates.[26-49] The higher response rates are for patients with platinum-sensitive disease. These agents are appropriate for patients with platinum-sensitive recurrent ovarian cancer who have had progression of disease on platinum-based second-line therapy.
Bevacizumab, a humanized monoclonal antibody that targets vascular endothelial growth factor, has been studied in several phase II studies alone and in combination with cytotoxic chemotherapy. It has been shown to be an active agent in patients with recurrent ovarian cancer.[20-24] The risk of gastrointestinal complications, specifically perforations, has been estimated to be 5% to 7%.[20-22] Some have suggested that bevacizumab should be used only in patients without clinical symptoms of bowel obstruction, CT scan evidence of bowel involvement, or evidence of rectosigmoid involvement on pelvic examination.[26] The role of bevacizumab in the management of epithelial ovarian cancer is evolving, with two phase III studies of upfront treatment and the OCEANS study in platinum-sensitive disease showing an improved PFS when bevacizumab is used with chemotherapy and as maintenance therapy. However, these trials have failed to show an OS advantage. Thus, for patients with recurrent platinum-sensitive disease, it may be more appropriate to use combination chemotherapy alone as second-line therapy, rather than combination chemotherapy plus bevacizumab. Bevacizumab may be appropriate to use in combination with single-agent therapy for subsequent lines of treatment or as single-agent therapy.
Maintenance therapy in platinum-sensitive recurrent ovarian cancer
Several clinical trials have evaluated the role of agents aimed at prolonging the second remission. One of these is the OCEANS trial, evaluating the role of bevacizumab, as noted above, in combination with chemotherapy and as maintenance therapy.
In a trial of 296 women with platinum-sensitive recurrent ovarian cancer who achieved a response to their most recent line of therapy, patients were randomized to placebo or the poly ADP-ribose polymerase (PARP) inhibitor olaparib (at 400 mg orally twice a day).[50] An improved PFS benefiting patients receiving olaparib was reported (8 vs 5 months; HR = 0.35; 95% CI, 0.25–0.49).[50] At interim analysis, there was no OS difference (30 months in both; HR = 0.94; 95% CI, 0.63–1.39).[50] Olaparib was associated with a higher rate of adverse events of all grades, including nausea (68% vs 35%), fatigue (49% vs 38%), vomiting (32% vs 14%), and anemia (17% vs 5%).[50]
Other agents are the subject of ongoing clinical trials. The folate receptor is overexpressed in approximately 90% of ovarian cancers. Farletuzumab, a humanized monoclonal antibody to the folate receptor alpha, has been the subject of a phase II study. Patients with platinum-sensitive recurrent ovarian cancer were treated with either single-agent farletuzumab or, if symptomatic, a combination regimen of paclitaxel, carboplatin, and farletuzumab. The reported overall response was 70%, with a median PFS of 10.9 months.[51] In a phase III trial, women with measurable, platinum-sensitive recurrent ovarian cancer were randomized to paclitaxel and carboplatin with or without farletuzumab. Farletuzumab in combination with carboplatin and a taxane did not meet the study’s primary endpoint of PFS. In the post hoc exploratory analysis, a trend toward improved PFS in some patient subsets was noted. Further analysis of the findings is ongoing.[52]
Another agent, iniparib, may be an irreversible PARP inhibitor potentially with other cytotoxic actions. In a phase II trial, iniparib, given in combination with carboplatin and gemcitabine, resulted in a 65% response rate and a PFI of 9.5 months in 41 women with platinum-sensitive recurrent ovarian cancer.[53]
Platinum-Resistant Recurrent Ovarian Cancer
Treatment selection
In general, patients with platinum-resistant recurrent ovarian cancer are treated with non–platinum-based chemotherapy. The goals and timing of the institution of therapy should focus on palliating cancer-related symptoms, prolonging life, and optimizing quality of life. In patients with platinum-resistant ovarian cancer, response rates are low and prognosis is generally poor. Non–cross-resistant agents should be considered, and the risk of overlapping toxicities, based on side effects from previous therapies, should be avoided. In general, higher response rates and longer 2- to 3-month PFS rates are seen with the use of combination regimens. However, combination therapy is associated with higher toxicity and no improvement in OS compared with single-agent therapy. Thus, for the treatment of platinum-resistant disease, we usually prefer single-agent therapy since it may offer a potential balance between efficacy of treatment and an acceptable toxicity profile.
Taxanes
Several drugs have documented activity in platinum-resistant disease. In phase II and III trials, single-agent paclitaxel has resulted in objective responses in 22% to 30% of patients.[27-30] Several dosing and scheduling regimens for paclitaxel have been investigated. Table 4 describes some of them.[54-57] Single-agent docetaxel is more toxic and less effective than paclitaxel.[46,47] In a phase II trial of 60 patients with platinum-resistant disease, a 22% objective response rate (RR) was seen, with a 2.5-month median response duration.[46] Grade 4 neutropenia was reported in 75% of docetaxel-treated women.[46]
Pegylated liposomal doxorubicin
In a phase III trial, PLD was compared with topotecan in women with recurrent ovarian cancer, following one platinum-containing regimen.[35,36] Patients were stratified prior to being randomized, according to their tumor’s platinum sensitivity. A similar overall RR (20% vs 17%), time to progression (22 vs 20 weeks) and median OS (66 vs 56 weeks) was documented with each regimen.[35] A significant OS advantage was noted at longer follow-up among patients treated with PLD, especially for those with platinum-sensitive disease (HR = 0.82; 95% CI, 0.68–1.0).[36] When compared with topotecan, PLD was associated with lower rates of neutropenia, anemia, and thrombocytopenia, and with higher rates of hand-foot syndrome and stomatitis.[36] Preliminary review of data from a phase III trial comparing PLD vs paclitaxel indicates that the regimens were similar in terms of response rates, PFI, and OS.[37] Hand-foot syndrome was seen in 20% of patients treated with PLD at 50 mg/m2 every 4 weeks.[38] Similar efficacy with lower toxicity has been seen at lower doses (35–40 mg/m2 every 4 weeks).[39,58]
Topotecan
Topotecan has similar efficacy to paclitaxel and PLD in the treatment of platinum-resistant recurrent ovarian cancer.[59] Its use is usually associated with some degree of myelosuppression, especially neutropenia.[35,60-67] Concomitant use of amifostine with topotecan in heavily pretreated patients has been reported to lower the risk of hematologic toxicity.[68]
Gemcitabine
Gemcitabine, approved in combination with carboplatin for treatment of platinum-sensitive recurrent ovarian cancer, has also been studied as single-agent therapy in the setting of platinum-resistant disease. In a study of 50 patients with recurrent ovarian cancer, gemcitabine, at 800 mg/m2 weekly for 3 out of every 4 weeks, was associated with a 19% RR.[31] In a nonequivalency trial comparing gemcitabine to PLD, no significant differences were seen between the two agents with respect to overall RR (6.1% vs 8.3%), median PFS (3.6 vs 3.1 months), and median OS (12.7 vs 13.5 months).[31] Gemcitabine in combination with PLD appears to be a well-tolerated active regimen. In a study of 31 patients with platinum-resistant or refractory disease, a 33% objective RR was seen with this combination.[33] Grade 3 or 4 neutropenia was seen in 25% of patients, underscoring the previously noted recommendation to consider single-agent therapy in the setting of platinum-resistant recurrence.
Other agents
Table 5 lists some of the other single-agent chemotherapy options appropriate for treating platinum-resistant disease.[42-49] Importantly, the recently presented AURELIA trial showed an improved PFS in patients with platinum-resistant ovarian cancer treated with bevacizumab in combination with single-agent chemotherapy when compared to treatment with chemotherapy alone (5.7 vs 4 months).[69]
Conclusions
Recurrent ovarian cancer is not curable. The goals of therapy should focus on palliation of cancer-related symptoms, extension of life, and maintenance of quality of life. Recurrent ovarian cancer is commonly dichotomized as platinum-sensitive or platinum-resistant. This definition, although limited, may be helpful for clinical trial purposes and in guiding treatment selection. However, it does have limitations.
In general, patients with platinum-sensitive ovarian cancer should have their recurrence treated with a platinum-based agent. Platinum-containing combination regimens are associated with higher response rates and PFS. Patients treated with paclitaxel in combination with carboplatin have been reported to have an improved OS compared with carboplatin alone. The choice of single-agent vs combination therapy should take into account the patients’ performance status, other comorbidities, and previous toxicities with other therapies. If combination therapy is elected, the specific combination regimen chosen should also take these factors into consideration, to optimize outcome and minimize overlapping toxicities.
For patients whose cancer progresses after platinum retreatment and for those with platinum-resistant disease, numerous other agents have been shown to be effective in palliating cancer-related symptoms and extending life. Future management strategies may include use of nonplatinum combination and targeted therapies, given alone or in combination with cytotoxic agents.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Siegel R, Naishadham MA, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10-29.
2. Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med. 1990;322:1021-7.
3. Thigpen JT, Vance RB, Khansur T. Second-line chemotherapy for recurrent carcinoma of the ovary. Cancer. 1993;71(4 suppl):1559-64.
4. Markman M, Markman J, Webster K, et al. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol. 2004;22:3120-5.
5. Pujade-Lauraine E, Paraiso D, Cure H, et al. Predicting the effectiveness of chemotherapy (Cx) in patients with recurrent ovarian cancer (ROC): a GINECO study. J Clin Oncol. 2002;21:Abstr 829.
6. Santillan A, Garg R, Zahurak ML, et al. Risk of epithelial ovarian cancer recurrence in patients with rising serum CA-125 levels within the normal range. J Clin Oncol. 2005;23:9338-43.
7. Liu PY, Alberts DS, Monk BJ, et al. An early signal of CA-125 progression for ovarian cancer patients receiving maintenance treatment after complete clinical response to primary therapy. J Clin Oncol. 2007;
25:3615-20.
8. Vergote I, Rustin GJ, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J Natl Cancer Inst. 2000;92:1534-5.
9. Doyle C, Crump M, Pintilie M, Oza AM. Does palliative chemotherapy palliate? Evaluation of expectations, outcomes, and costs in women receiving chemotherapy for advanced ovarian cancer. J Clin Oncol. 2001;19:1266-74.
10. Cantu MG, Buda A, Parma G, et al. Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubucin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. J Clin Oncol. 2002; 20:1232-7.
11. Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991; 9:389-93.
12. Ferrandina G, Ludovisi M, De Vicenzo R, et al. Docetaxel and oxaliplatin in the second-line treatment of platinum-sensitive recurrent ovarian cancer: a phase II study. Ann Oncol. 2007;18:1348-53.
13. Canistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519-29.
14. Rustin GJ, van der Burg ME, et al. A randomized trial in ovarian cancer (OC) or early treatment of relapse based on CA125 level alone versus delayed treatment based on conventional clinical indicators (MRC OV05/EORTC 55955 trials). J Clin Oncol. 2009; 27(18 suppl):Abstr 1.
15. Cantù MG, Buda A, Parma G, et al. Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. J Clin Oncol. 2002;20:1232-7.
16. Cannistra SA. Is there a “best” choice of second-line agent in thetreatment of recurrent, potentially platinum-sensitive ovarian cancer? J Clin Oncol. 2002; 20:1158-60.
17. Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2-2 trial. Lancet. 2003;361:2099-2106.
18. Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699-4707.
19. Pujade-Lauraine E, Mahner S, Kaem J, et al. A randomized, phase III study of carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in relapsed platinum-sensitive ovarian cancer (OC). CALYPSO study of the Gynecologic Cancer Intergroup (GCIG). J Clin Oncol. 2009;27:(18 suppl): Abstr LBA5509.
20. Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a GOG study. J Clin Oncol. 2007;25:5165-71.
21. Garcia AA, Hirte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital Phase II consortia. J Clin Oncol. 2008;26:76-82.
22. Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180-6.
23. Aghajanian C, Blank S , Goff B, et al. Results from a 2nd interim OS analysis in OCEANS, a randomized phase 3 trial of gemcitabine (G), carboplatin (C) and bevacizumab (BV) followed by BV to disease progression in patients with platinum-sensitive recurrent epithelial ovarian (OC), primary peritoneal (PPC), or fallopian tube cancer (FTC). Presented at the 2012 SGO Annual Meeting, Austin, TX: Abstr 2289.
24. Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blinded, placebo-controlled phase III trial of chemotherapy with or without bevacizumab (BEV) in patients with platinum-sensitive recurrent epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC). J Clin Oncol. 2012;30:2039.
25. Monk BJ, Herzog T, Kaye S, et al. A randomized phase III study of trabectedin with pegulated liposomal doxorubicin (PLD) versus PLD in relapsed, recurrent ovarian cancer. Proceedings of the 33rd ESMO Congress, Stockholm, Sweden, 2008:Abstr LBA4.
26. Simpkins F, Belinson JL, Rose PG. Avoiding bevacizumab related gastrointestinal toxicity for recurrent ovarian cancer by careful patient screening. Gynecol Oncol. 2007;107:118-23.
27. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian adenocarcinoma. Ann Intern Med. 1989;111:273-9.
28. Einzig AI, Wiernik PH, Sasloff J, et al. Phase II study and long-term follow-up of patients treated with Taxol for advanced ovarian adenocarcinoma. J Clin Oncol. 1992;10:1748-53.
29. Thigpen JT, Blessing JA, Ball H, et al. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a GOG study. J Clin Oncol. 1994;12:1748-53.
30. Kohn EC, Sarosy G, Bicher A, et al. Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. J Natl Cancer Inst. 1994;86:18-24.
31. Lund B, Hansen OP, Theilade K, et al. Phase II study of gemcitabine (2’,2’-difluorodeoxycytidine) in previously treated ovarian cancer patients. J Natl Cancer Inst. 1994;86:1530-3.
32. Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared to pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811-8.
33. Petru E, Angleitner-Boubenizek L, Reinthaller A, et al. Combined PEG liposomal doxorubicin and gemcitabine are active and have acceptable toxicity in patients with platinum-refractory and –resistant ovarian cancer after previous platinum-taxane therapy: a phase II Austrian AGO study. Gynecol Oncol 2006;102:226-9.
34. Krasner C, Poveda A, Herzog TJ, et al. Patient-reported outcomes in relapsed ovarian cancer: results from a randomized phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD alone. Gynecol Oncol. 2012; Jul 2. [Epub ahead of print]
35. Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312-22.
36. Gordon AN, Teitelbaum A. Overall survival advantage for pegylated liposomal doxorubicin compared to topotecan in epithelial ovarian cancer. Eur J Cancer. 2003;12:Abstr S51.
37. O’Byrne KJ, Bliss P, Graham JD, et al. A phase III study of Doxil/Caelyx versus paclitaxel in platinum-treated, taxane-naive relapsed ovarian cancer. Proc Am Soc Clin Oncol. 2002;21:Abstr 808.
38. Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol. 1997;15:987-93.
39. Rose PG, Maxson JH, Fusco N, et al. Liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a retrospective comparative study of single-agent dosages. Gynecol Oncol. 2001;82:323-8.
40. Kudelka AP, Tresukosol D, Edwards CL, et al. Phase II study of intravenous topotecan as a 5-day infusion for refractory epithelial ovarian carcinoma. J Clin Oncol. 1996;14:1552-7.
41. Hoskins P, Eisenhauer E, Beare S, et al. Randomized phase II study of two schedules of topotecan in previously treated patients with ovarian cancer: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol. 1998;16:2233-7.
42. Sorensen P, Hoyer M, Jakobsen A, et al. Phase II study of vinorelbine in the treatment of platinum-resistant ovarian carcinoma. Gynecol Oncol. 2001; 81:58-62.
43. Burger RA, DiSaia PJ, Roberts JA, et al. Phase II trial of vinorelbine in recurrent and progressive epithelial ovarian cancer. Gynecol Oncol. 1999;72:148-53.
44. Slayton RE, Creasman WT, Petty W, et al. Phase II trial of VP-16-213 in the treatment of advanced squamous cell carcinoma of the cervix and adenocarcinoma of the ovary: a Gynecologic Oncology Group study. Cancer Treat Rep. 1979;63:2089-94.
45. Rose PG, Blessing JA, Mayer AR, Homesley HD. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a GOG study. J Clin Oncol. 1998;16:405-10.
46. Rose PG, Blessing JA, Ball HG, et al. A phase II study of docetaxel in paclitaxel-resistant ovarian and peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:130-5.
47. Markman M, Zanotti K, Webster K, et al. Phase 2 trial of single agent docetaxel in platinum and paclitaxel-refractory ovarian cancer, fallopian tube cancer, and primary carcinoma of the peritoneum. Gynecol Oncol. 2003;91:573-6.
48. Markman M, Hakes T, Reichman B, et al. Ifosfamide and mesna in previously treated advanced epithelial ovarian cancer: activity in platinum-resistant disease. J Clin Oncol. 1992;10:243-8.
49. Miller DS, Blessing JA, Krasner CN, Mannel RJ. A phase II evaluation of pemetrexed (LY231514, IND#40061) in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecology Oncology Group. J Clin Oncol. 2008;26(suppl):Abstr 5524.
50. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382.
51. Armstrong DK. Exploratory phase 2 efficacy study of MORAb-003, a monoclonal antibody against folate receptor alpha, in platinum-sensitive epithelial ovarian cancer in first relapse. Presented at the Joint ECCO 15–34th ESMO Multidisciplinary Congress, Berlin, 2009.
52. Morphotek. Morphotek announces top-line results of a phase III study of farletuzumab in patients with relapsed platinum-sensitive epithelial ovarian cancer. Available here.
53. Penson RT, Whalen C, Lasonde B, et al. A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-sensitive recurrent ovarian cancer. J Clin Oncol. 2011;29:Abstr 5004.
54. Eisenhauer EA, ten Bokkel Huinin WW, Swenerton KD, et al. European-Candadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose verus low-dose and long versus short infusion. J Clin Oncol. 1994;12:2654-66.
55. Markman M, Hall J, Spitz D, et al. Phase II trial of weekly single-agent paclitaxel in platinum/paclitaxel-refactory ovarian cancer. J Clin Oncol. 2002;20:2365-9.
56. Kita L, Kikuchi Y, Takano M, et al. The effect of single weekly paclitaxel in heavily pretreated patients with recurrent or persistent advanced ovarian cancer. Gynecol Oncol. 2004;92:813-18.
57. Markman M, Blessing J, Rubin SC, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;101:436-40.
58. Markman M, Kennedy A, Webster K, et al. Phase 2 trial of liposomal doxorubicin (40 mg/m2) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol. 2000;78:369-72.
59. Peng L, Cheng X, Wu T. Topotecan for ovarian cancer. Cochrane Database Syst Rev. 2008;CD005589.
60. McGuire WP, Blessing JA, Bookman MA, et al. Topotecan has substantial antitumor activity as first-line salvage therapy in platinum-sensitive epithelial ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18:1062-7.
61. Bookman MA, Malmstrom H, Bolis G, et al. Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that combined cisplatin or carboplatin and paclitaxel. J Clin Oncol. 1998;16:3345-62.
62. Kudelka AP, Trresukosol D, Edwards CL, et al. Phase II study of intravenous topotecan as a 5-day infusion for refractory epithelial ovarian carcinoma. J Clin Oncol. 1996;14:1552-7.
63. Hoskins P, Eisenhauer E, Beare S, et al. Randomized phase II study of two schedules of topotecan in previously treated patients with ovarian cancer: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol. 1998;16:2233-7.
64. Brown JV, Peters WA, Rettenmaier MA, et al. Three-consecutive-day topotecan is an active regimen for recurrent epithelial ovarian cancer. Gynecol Oncol. 2003;88:136-40.
65. Herzog TJ, Powel MA, Rader JS, et al. Phase II evaluation of a 3-day infusion of topotecan in patients with recurrent ovarian or primary peritoneal cancer. Gynecol Oncol. 2006;103:637-41.
66. Safra T, Menczer J, Bernstein R, et al. Efficacy and toxicity of weekly topotecan in recurrent epithelial ovarian and primary peritoneal cancer. Gynecol Oncol. 2007;105:205-10.
67. Abushahin G, Singh DK, Lurain JR, et al. Weekly topotecan for recurrent platinum resistant ovarian cancer. Gynecol Oncol. 2008;108:53-7.
68. Gold MA, Walker JL, Berek JS, et al. Amifostine pretreatment for protection against topotecan-induced hematologic toxicity: results of a multicenter phase III trial in patients with advanced gynecologic malignancies. Gynecol Oncol. 2003;90:325-30.
69. Pujade-Lauraine E, Hilpert F, Weber B, et al. AURELIA: A randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). J Clin Oncol. 2012;30:Abstr LBA5002.